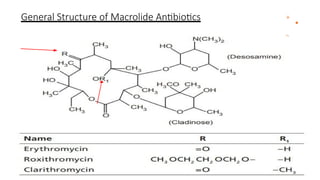
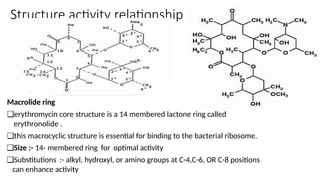
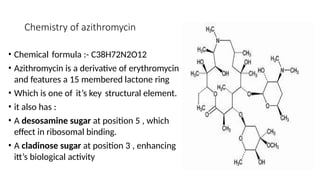
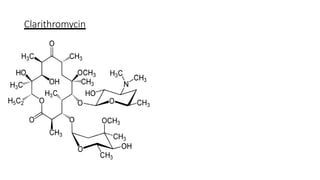
Macrolides are a class of broad-spectrum antibiotics produced by Streptomyces, characterized by a macrolide ring structure, and function primarily by inhibiting protein synthesis on the 50s ribosomal subunit. Significant examples include erythromycin, azithromycin, and clarithromycin, with azithromycin noted for its superior pharmacokinetics and reduced side effects. Recent research indicates that newer macrolides like azithromycin may provide enhanced antimicrobial activity and lower frequency of administration compared to older drugs.
















![Analogues of macrolide natural products
• Macrolide natural products with 12, 14, or 16 membered rings and their
semisynthetic derivatives are important clinically relevant compounds
predominantly used for the treatment of various types of cancer.
• Macrolactams are easily synthesized and offer access to multiple analogues
with chemical and enzymatic stability as well as desirable biological properties.
15-Aza-epothilone B
[Ixabepilone]](https://image.slidesharecdn.com/macrolidespresentations-250122190632-f4d7a71d/85/macrolides-Structure-activity-relation-ship-PRESENTATIONS-pptx-22-320.jpg)