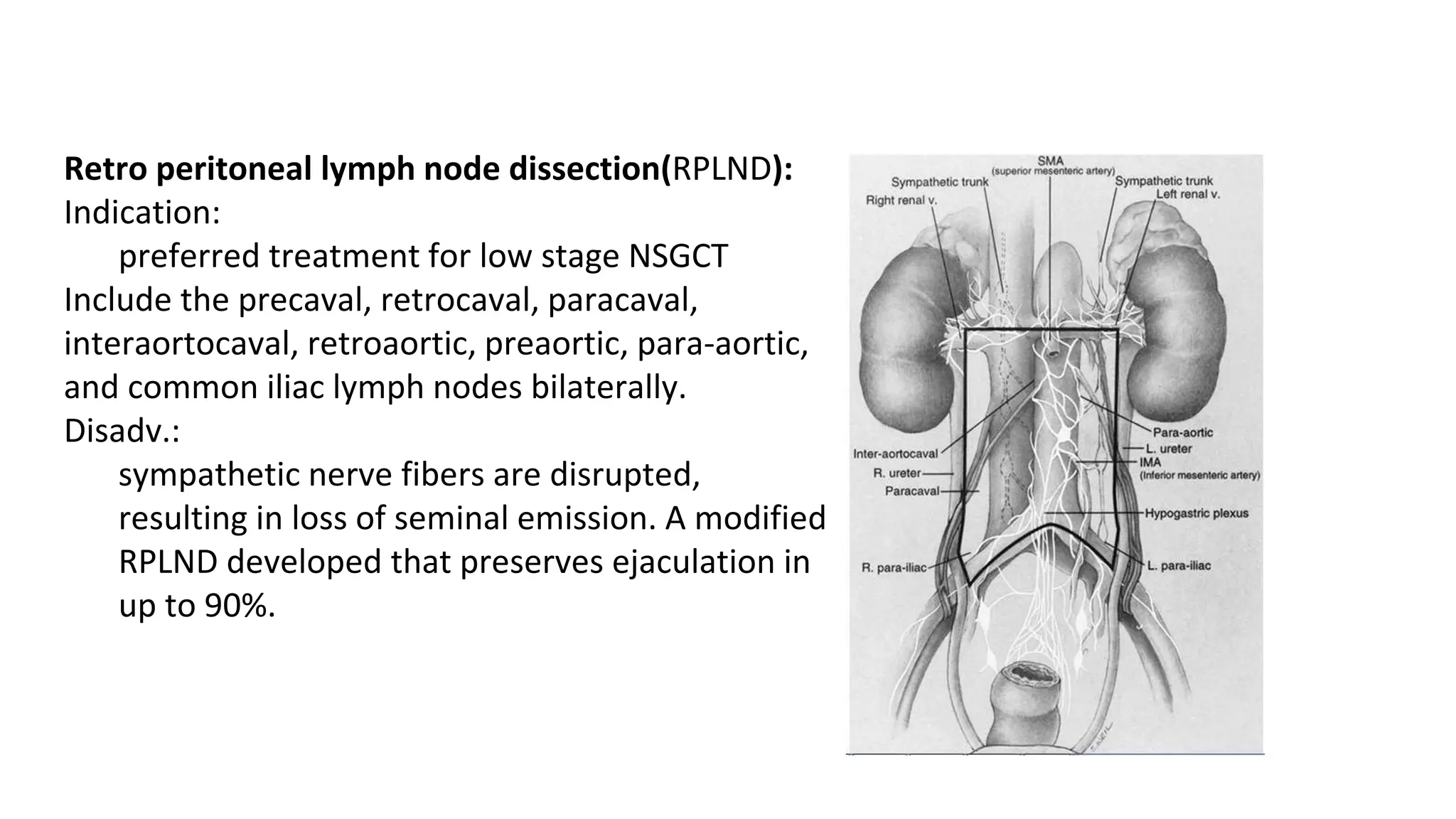
This document provides information about testicular cancers, including:
- Testicular cancer accounts for 1% of cancers in males and is highly curable when detected early, often affecting young men.
- The testis has blood supply from the testicular artery and drains into the pampiniform plexus and internal spermatic veins. Lymphatic drainage is to retroperitoneal lymph nodes.
- The majority (95%) are germ cell tumors, including seminomas and non-seminomas. Staging involves tumor markers, imaging scans, and lymph node dissection. Treatment depends on the type and stage but may include surgery, chemotherapy, and radiation therapy.