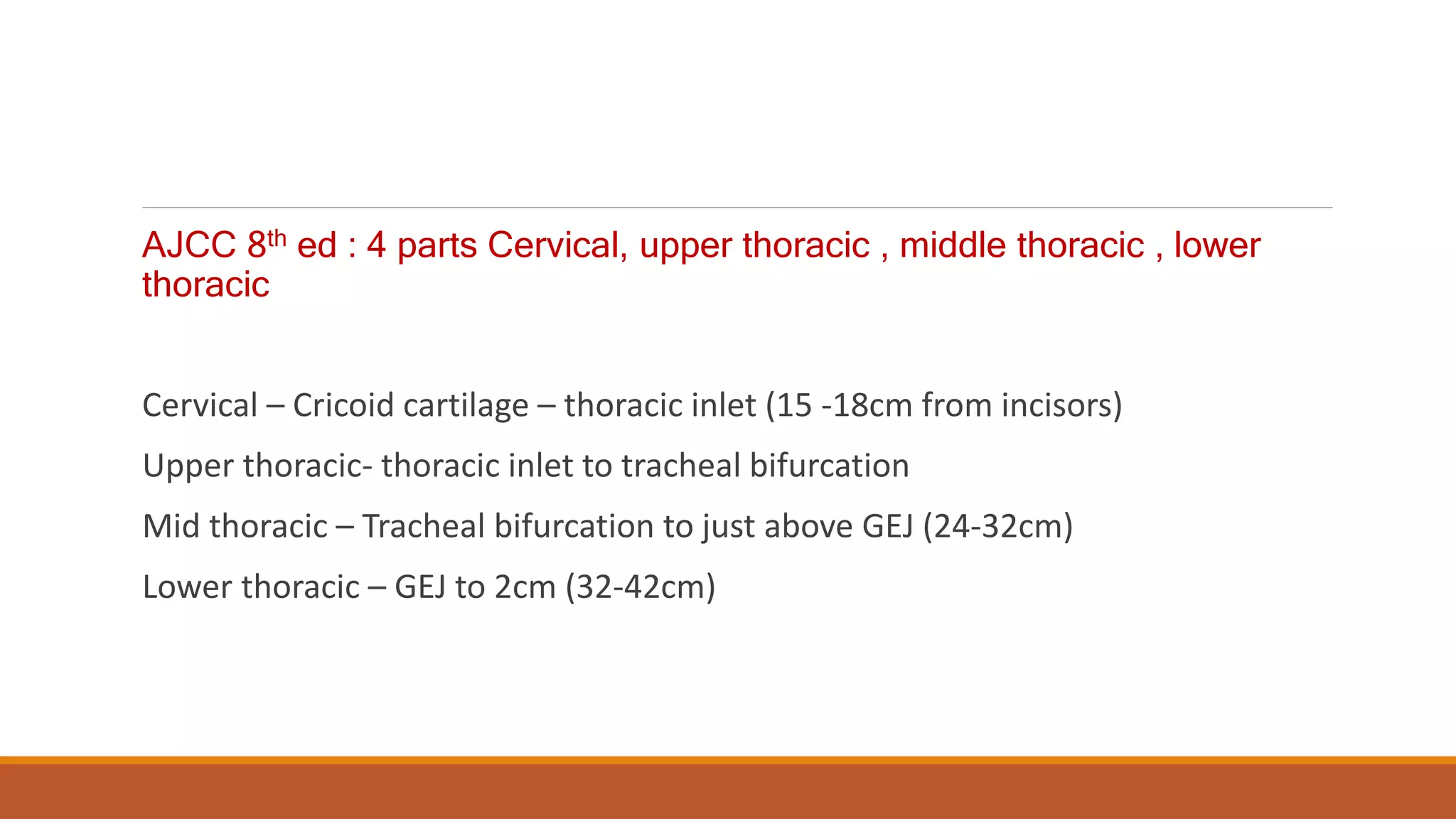
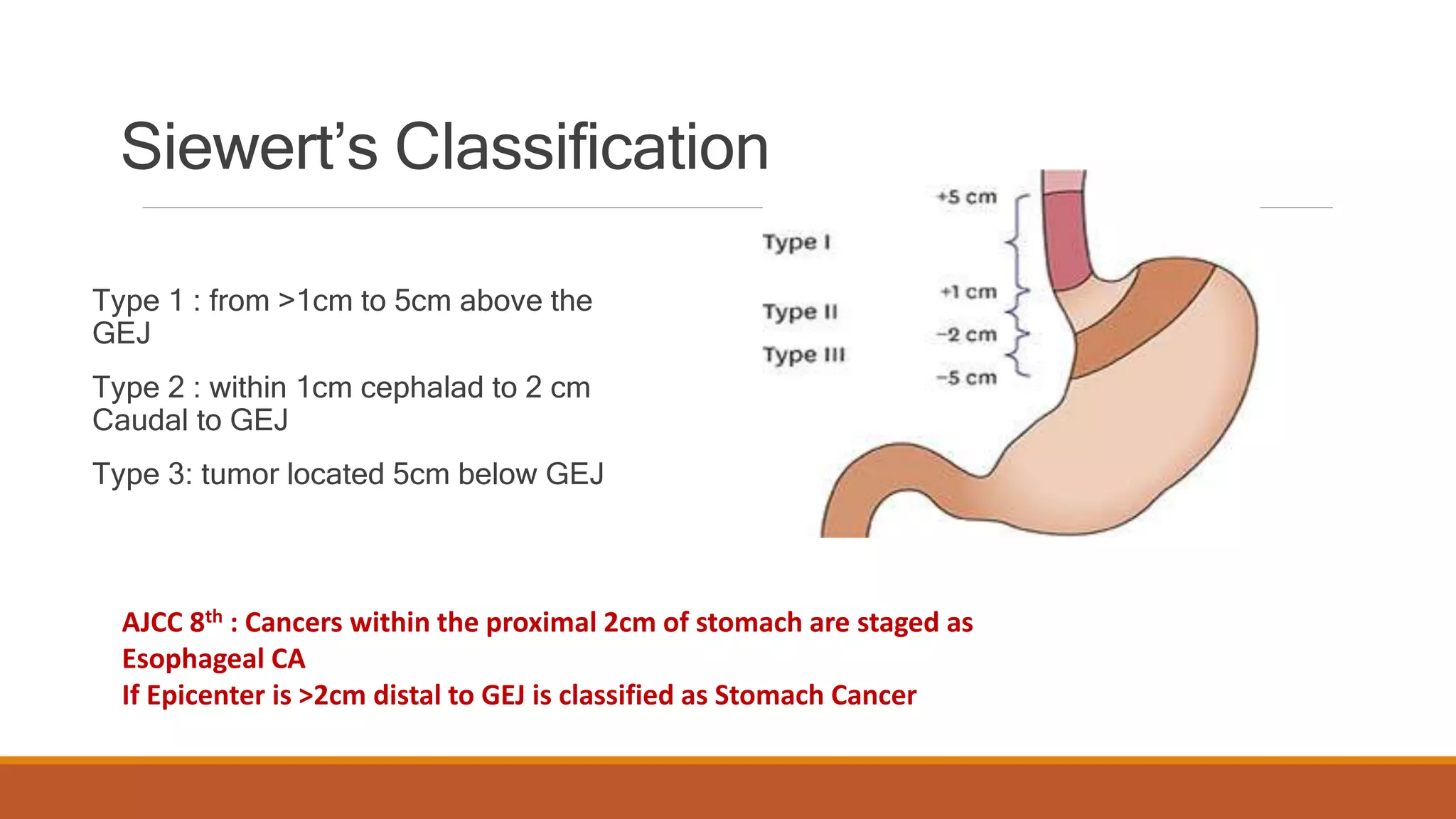
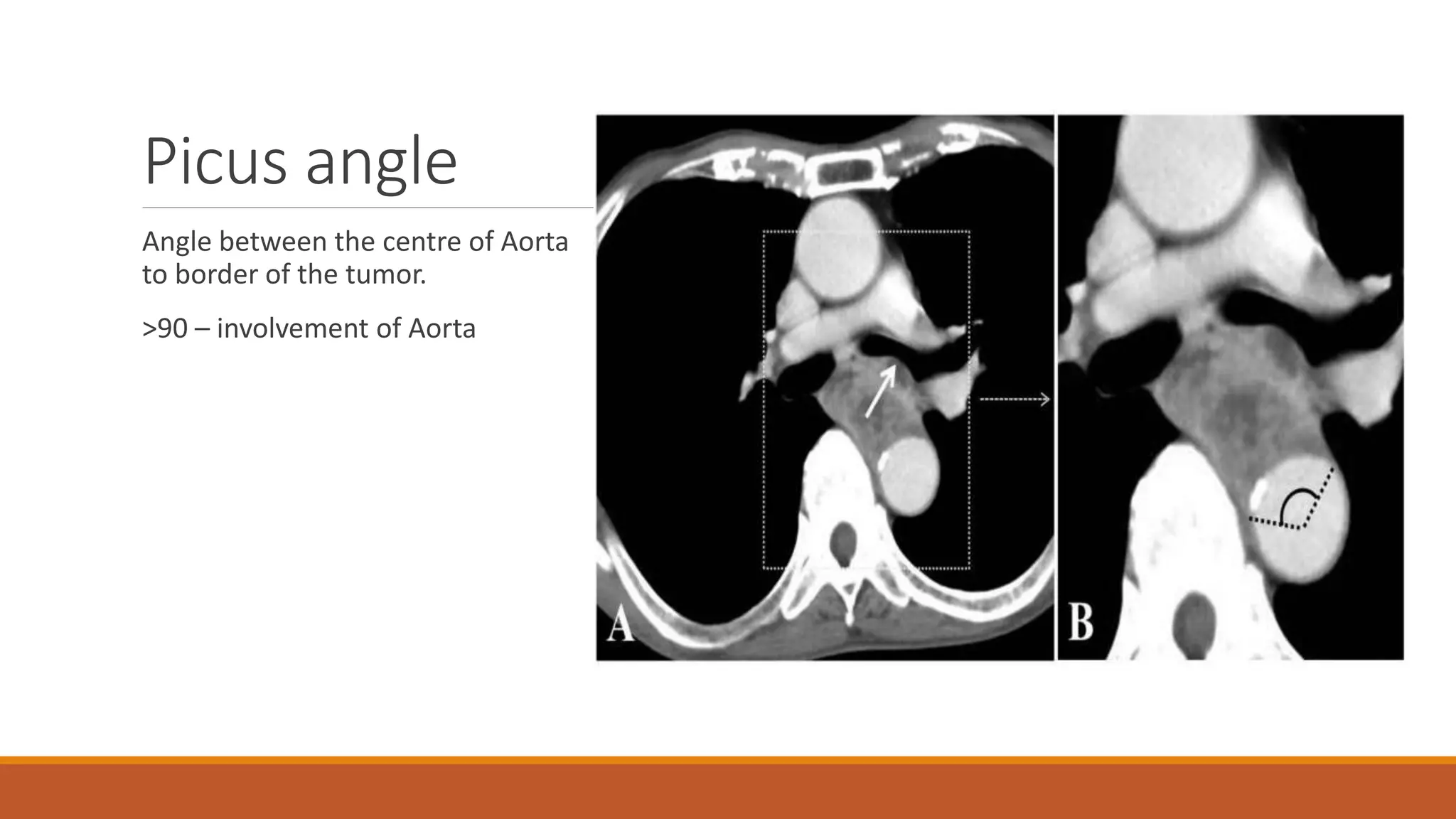
The document discusses carcinoma of the esophagus, including its anatomy, epidemiology, risk factors, staging, types, and management. It covers the various surgical techniques for esophagectomy, such as Ivor Lewis, McKeown, and transhiatal procedures. Post-operative care including drainage tube removal and diet progression is also summarized.