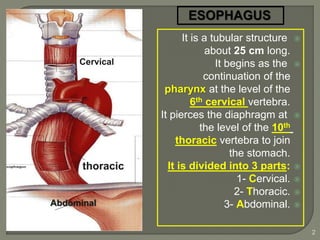
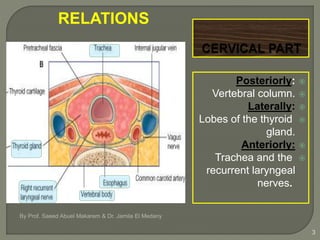
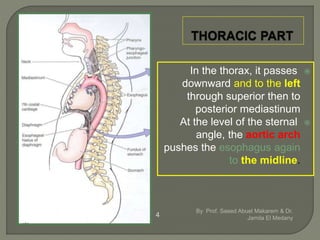
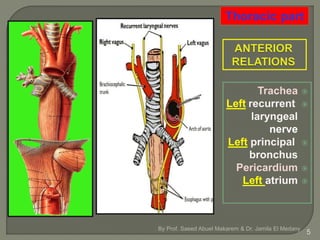
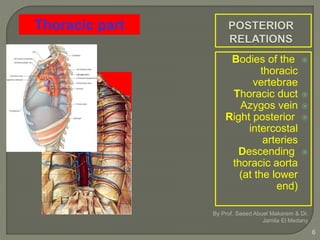
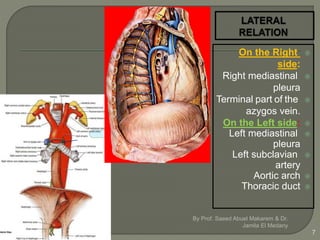
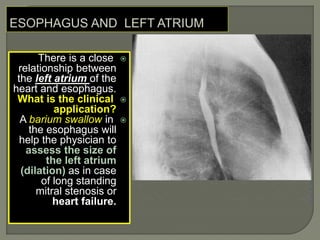
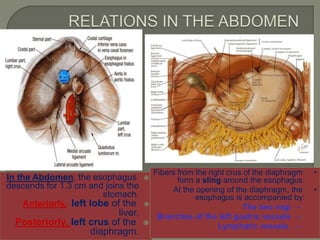
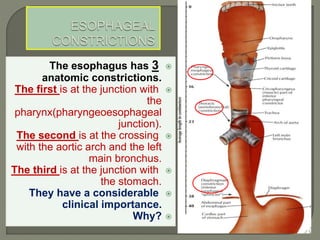
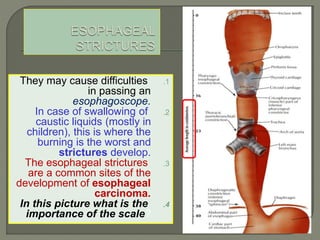
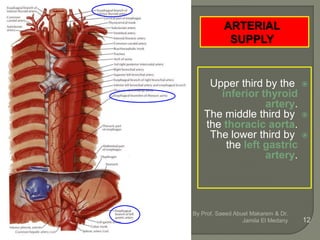
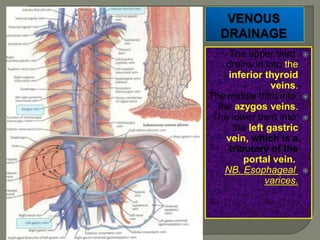
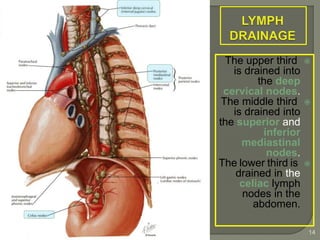
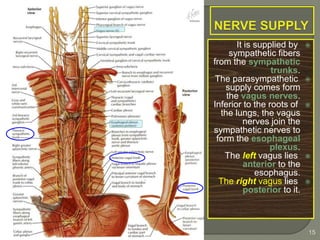
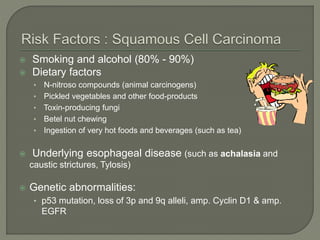
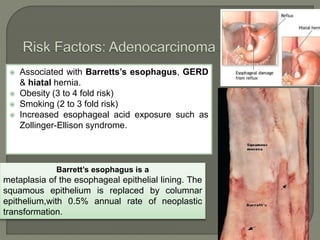
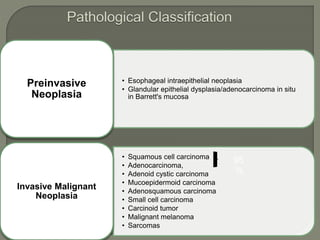
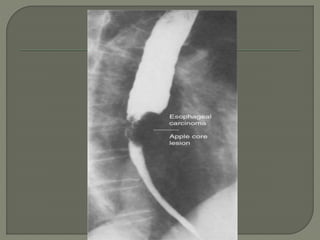
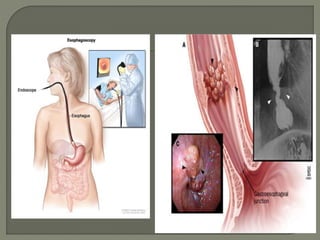
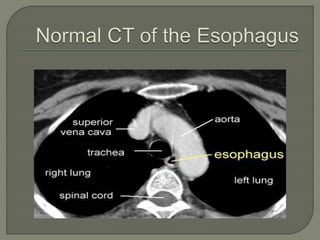
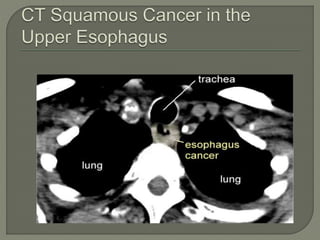
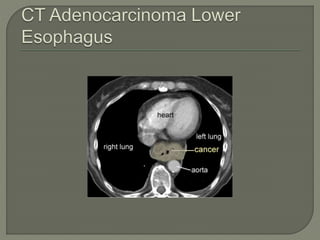
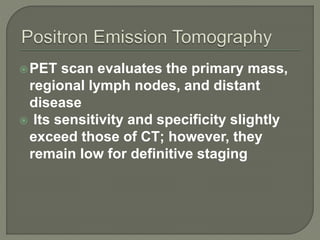
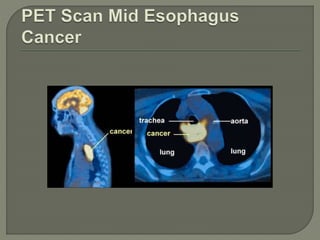
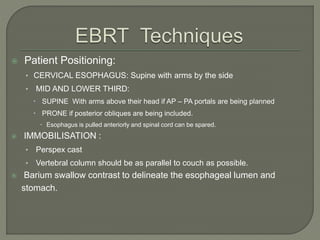
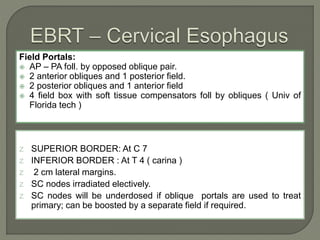
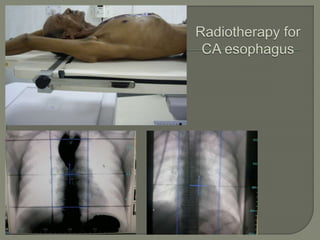
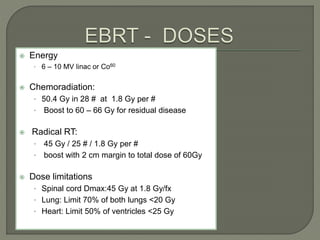
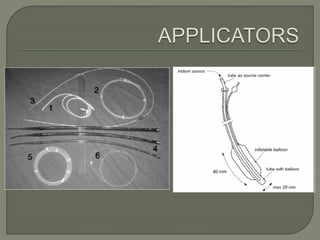
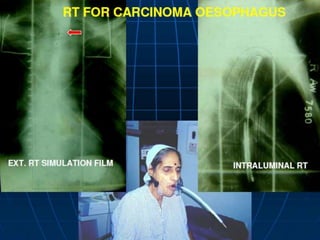
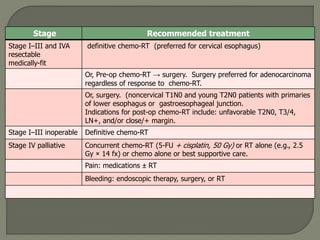
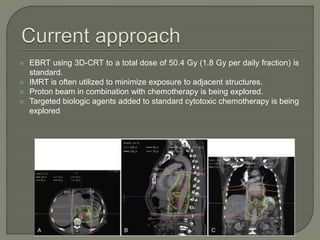
The document describes the anatomy and clinical aspects of the esophagus. It notes that the esophagus is a tubular structure about 25 cm long that begins at the pharynx and pierces the diaphragm to join the stomach. It discusses the relations of the esophagus in the neck, thorax, and abdomen. The document also summarizes the blood supply, lymphatic drainage, and innervation of the esophagus. Finally, it reviews esophageal cancer risk factors, staging, and treatment options including surgery, chemotherapy, and radiation therapy.