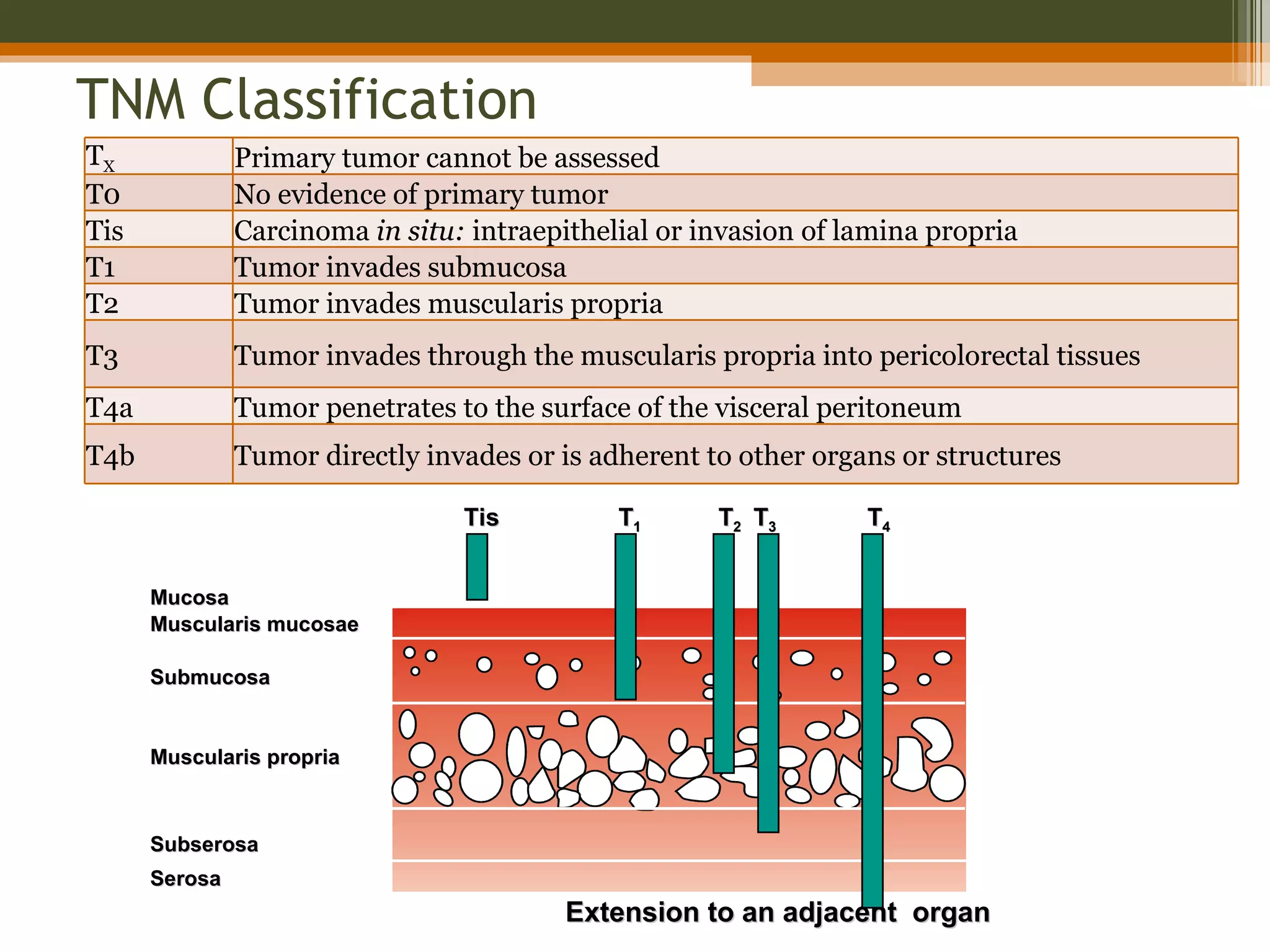
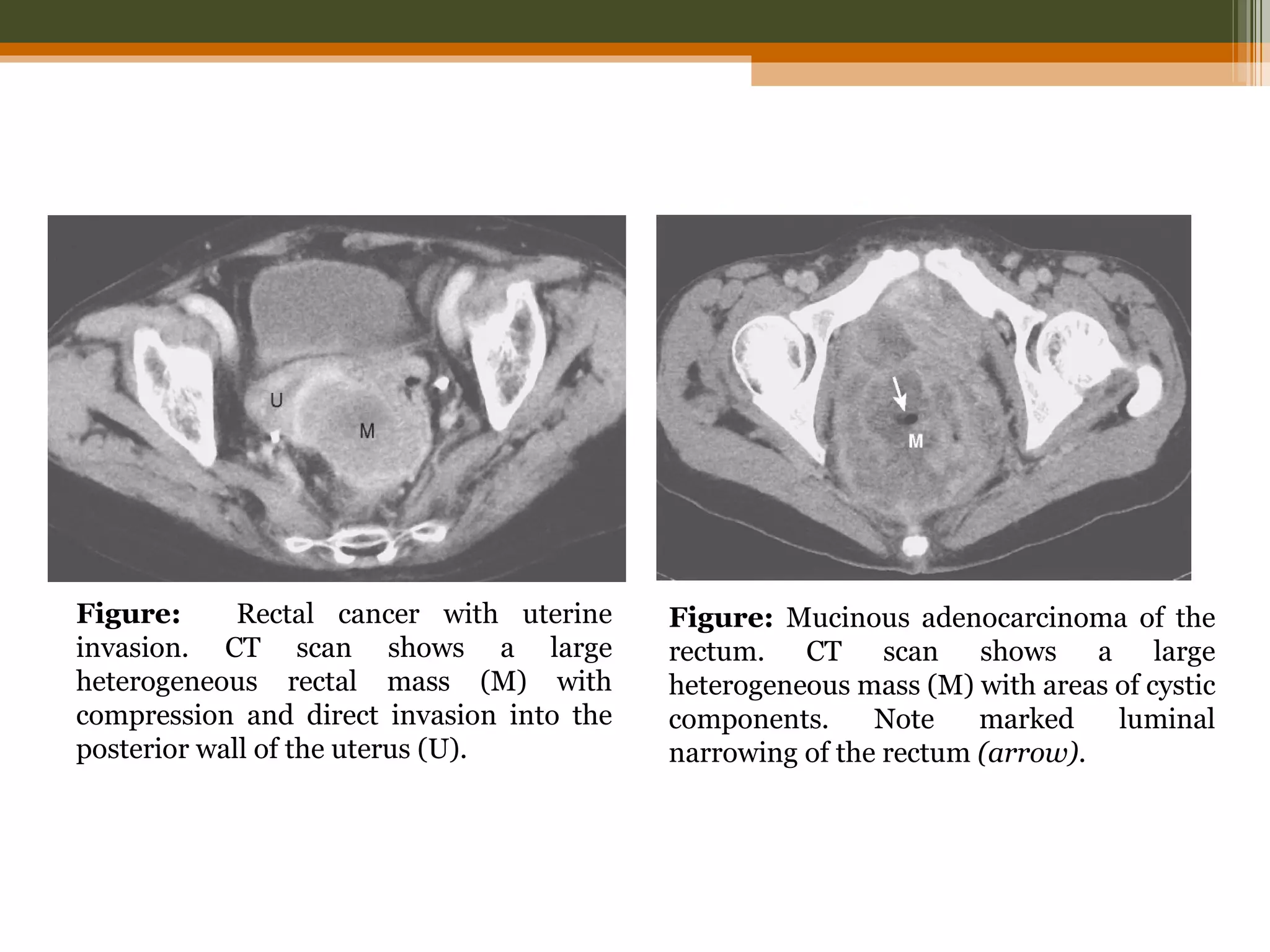
The document summarizes key anatomical and clinical aspects of the rectum:
1. The rectum is 12-15 cm long, located in the pelvis behind the lower sacrum and coccyx. It has three sections with varying peritoneal coverage and blood supply.
2. Rectal cancer is the third most common cancer in the US. Risk factors include diet, family history, and conditions like ulcerative colitis. Symptoms often include changes in bowel habits or bleeding.
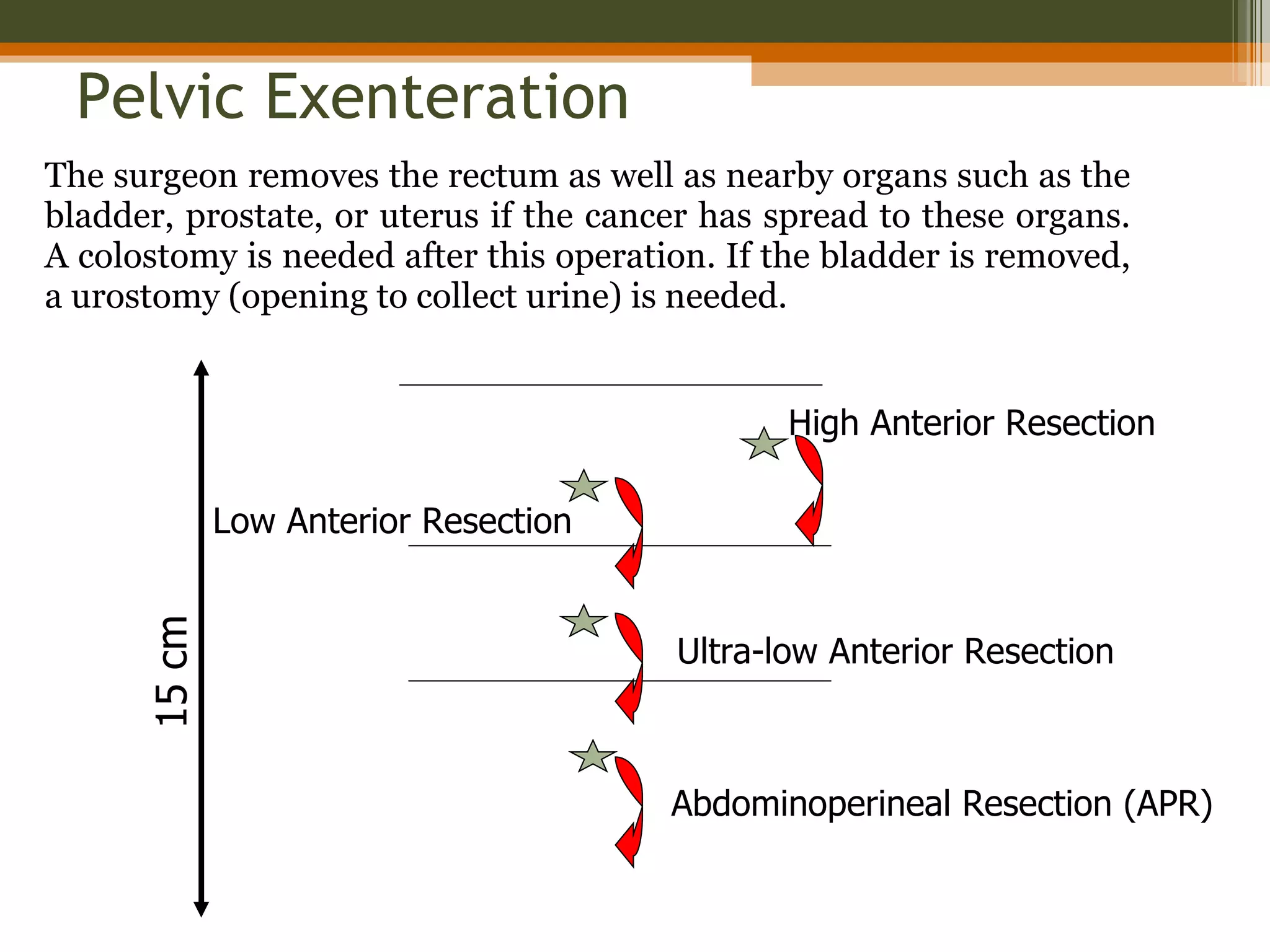
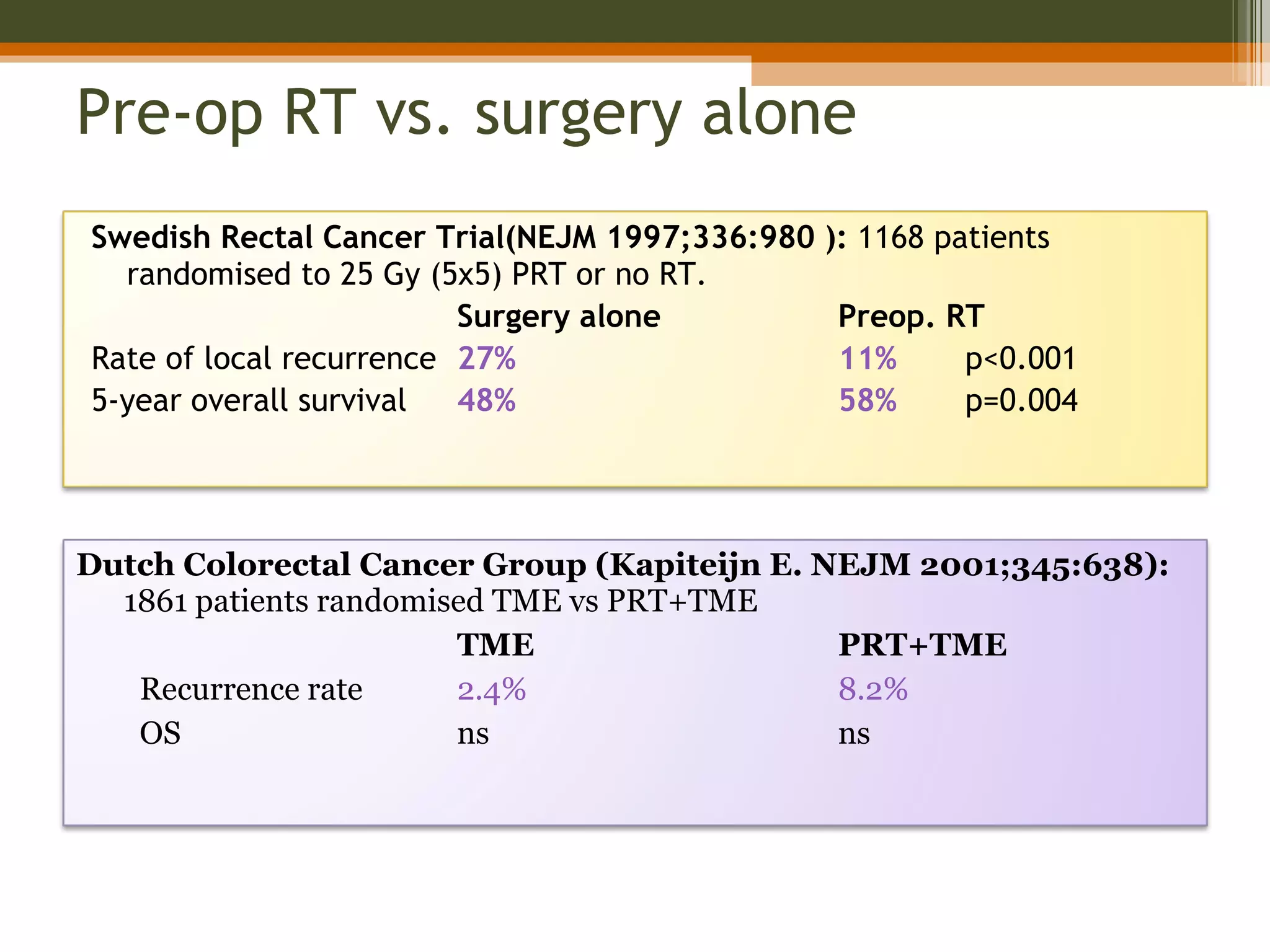
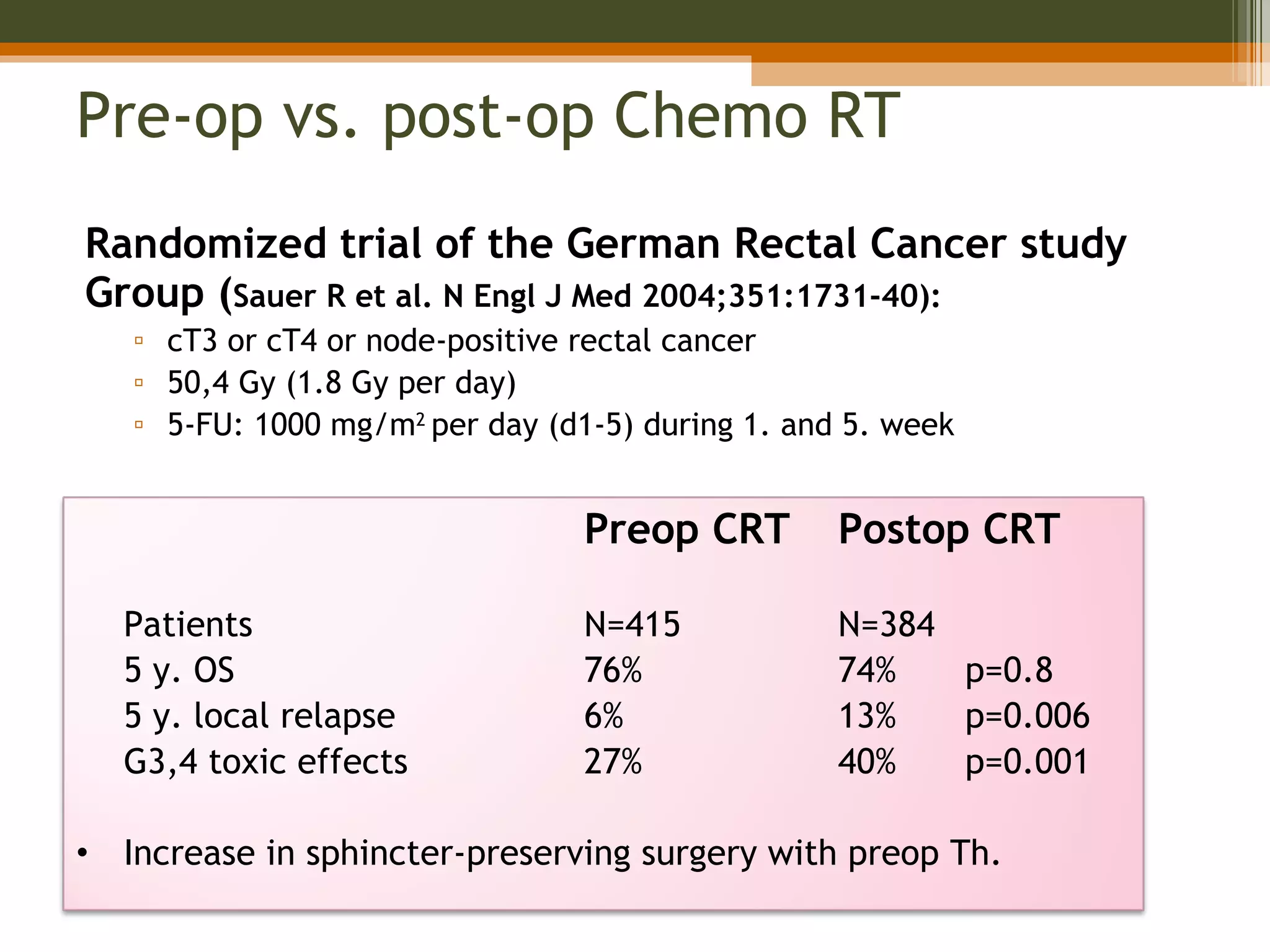
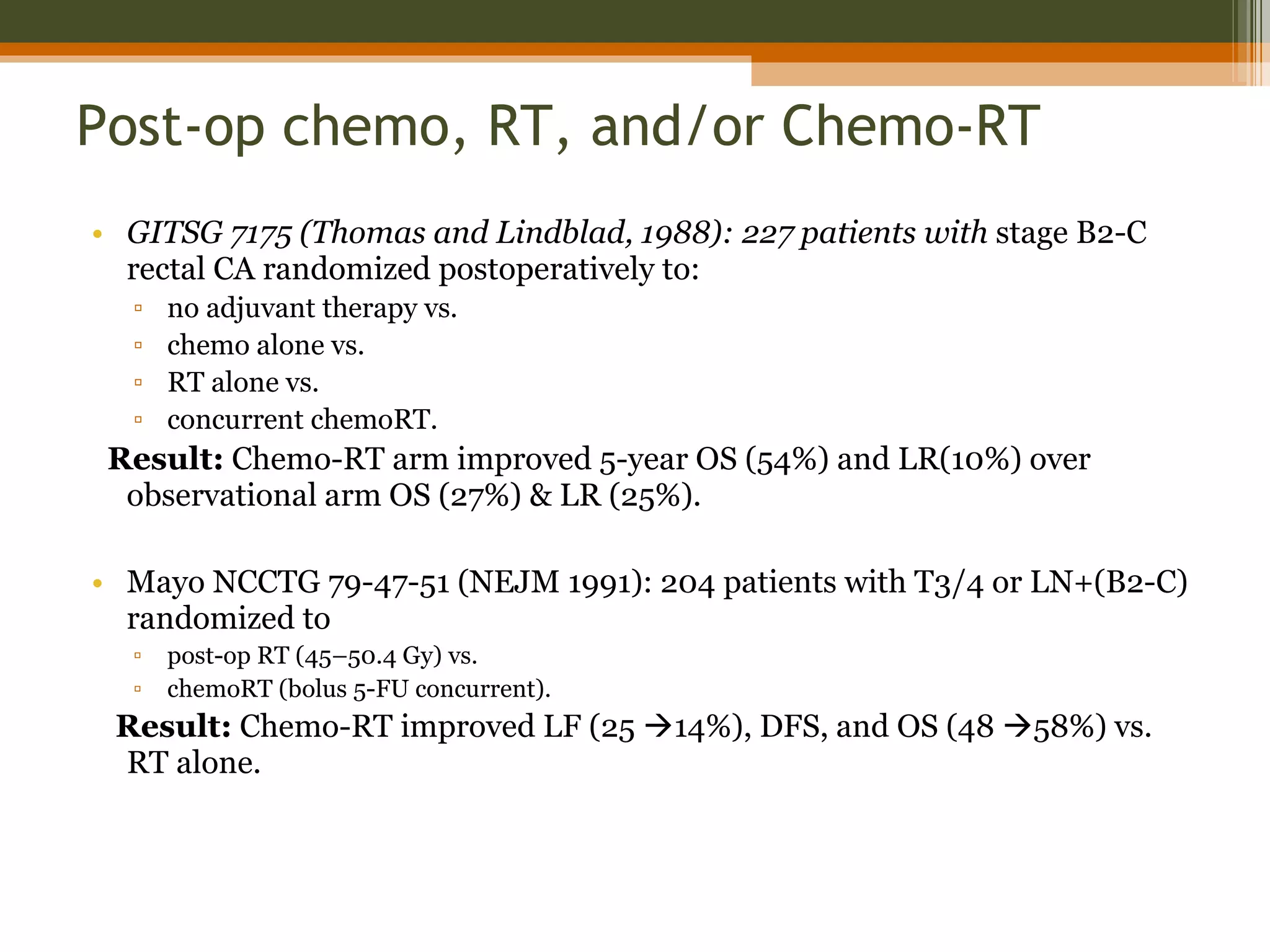
3. Treatment involves surgery like low anterior resection or abdominoperineal resection. Total mesorectal excision improves outcomes by completely removing the mesorectum and reducing local recurrence rates.