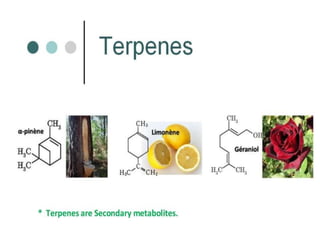
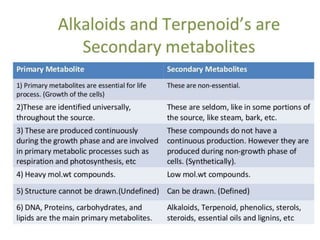
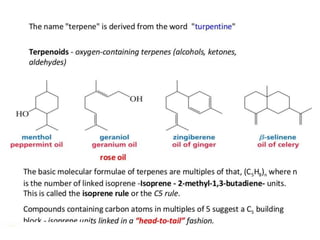
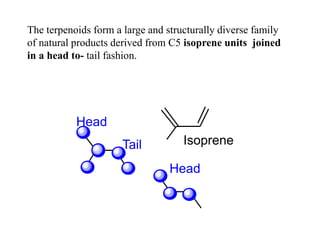
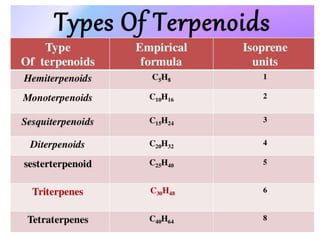
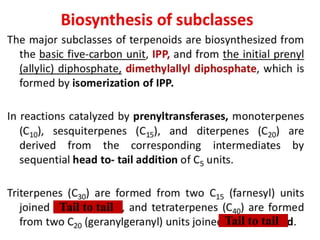
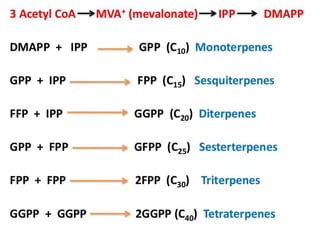
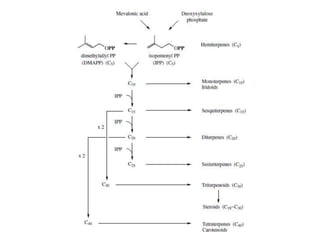
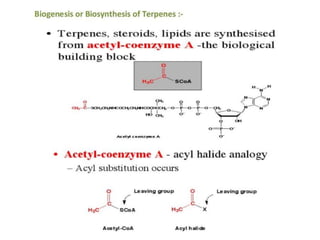
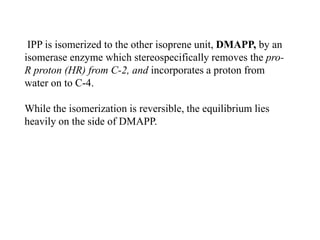
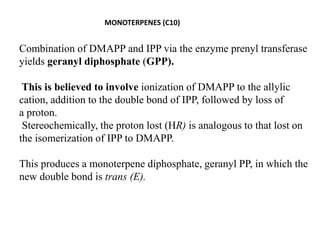
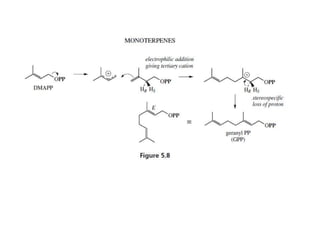
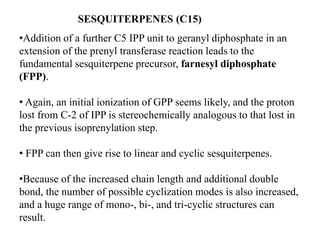
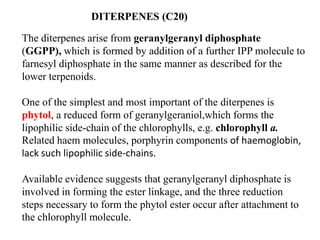
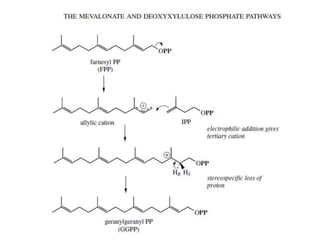
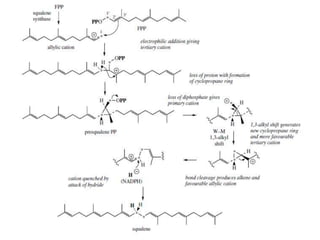
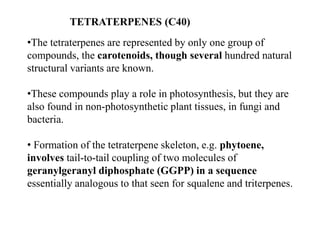
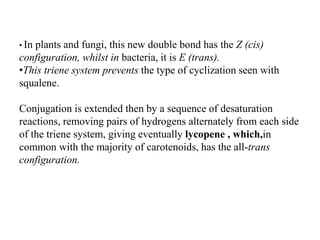
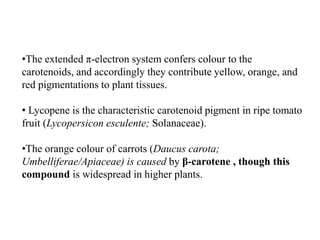
The document discusses the biosynthesis of terpenoids, classifying them based on their carbon chain lengths, from monoterpenes to tetraterpenes. Key processes include the isomerization of isoprene units to form precursors like geranyl, farnesyl, and geranylgeranyl diphosphates, leading to various natural products, including phytol and carotenoids. The document highlights the structural diversity and roles of these compounds in nature, particularly in photosynthesis and pigmentation.