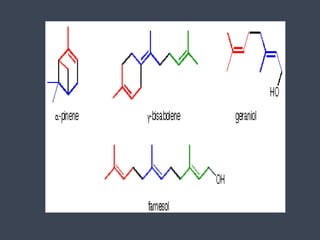
The document discusses terpenes and terpenoids. It defines terpenes as hydrocarbons found in plant essential oils and terpenoids as a subclass of prenyl lipids that represent the oldest group of small molecules synthesized by plants. Terpenoids are derived from isoprene units and are universally present in small amounts playing vital roles in plant physiology and cellular membranes. They display a large diversity that may be due to ecological factors playing an evolutionary role. The document then classifies and provides examples of different types of terpenoids including monoterpenoids, sesquiterpenoids, diterpenoids, sesterpenoids, triterpenoids, and others. It also discusses terpene biosynthesis and