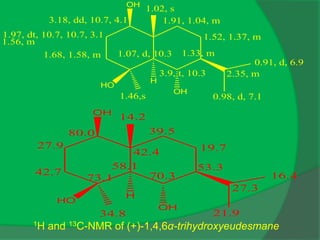
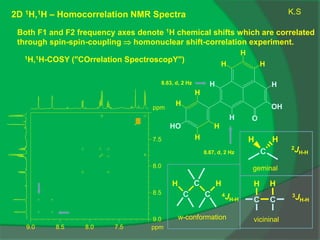
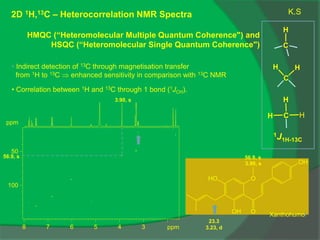
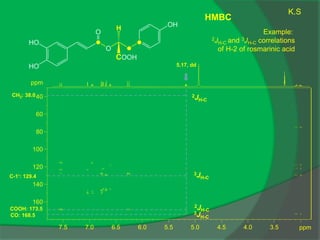
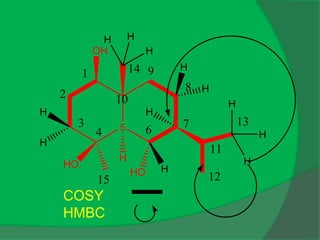
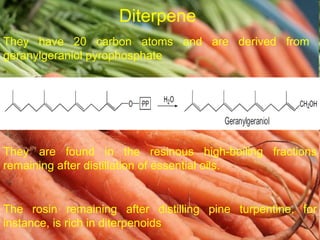
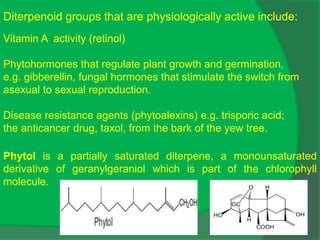
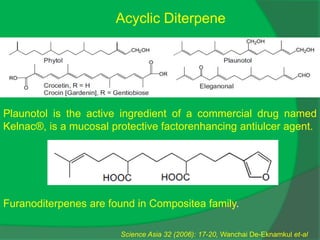
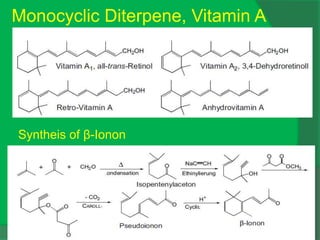
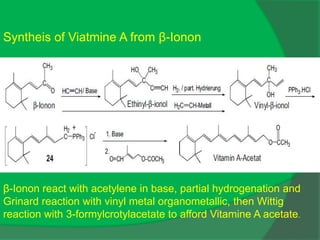
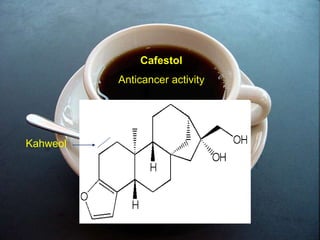
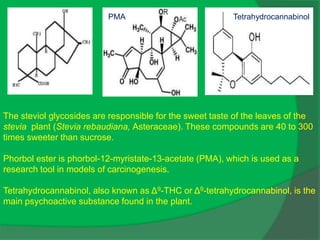
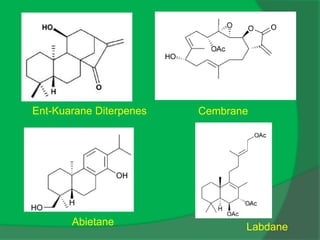
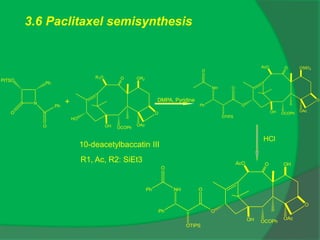
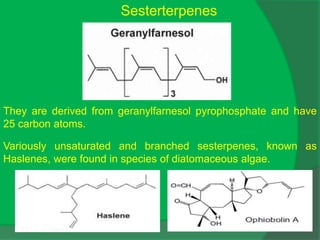
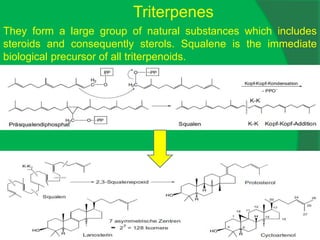
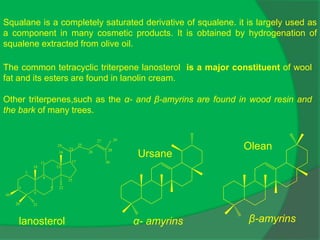
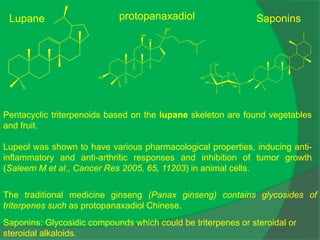
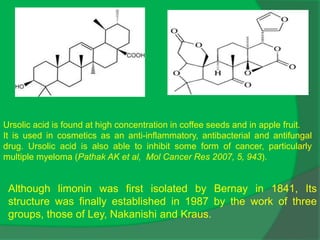
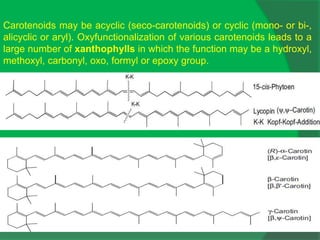
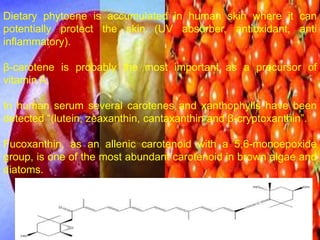
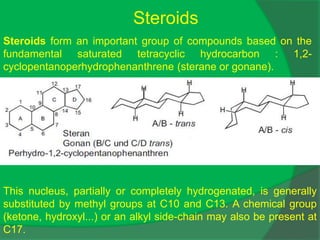
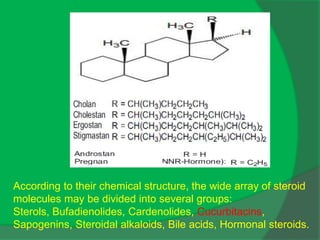
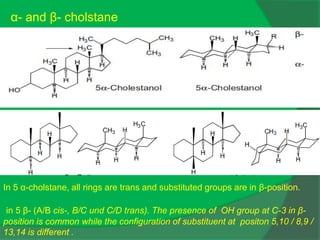
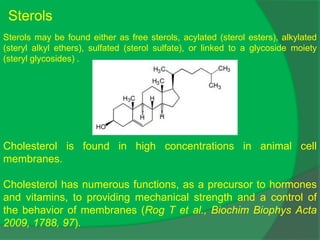
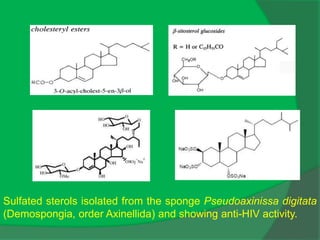
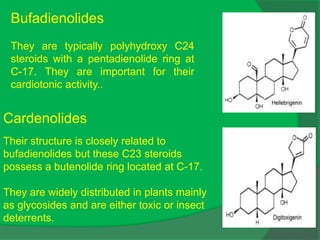
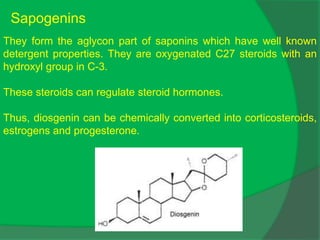
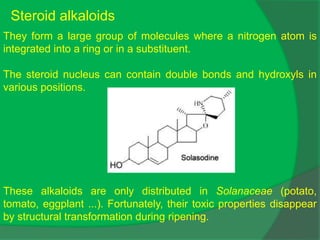
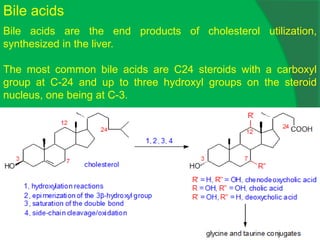
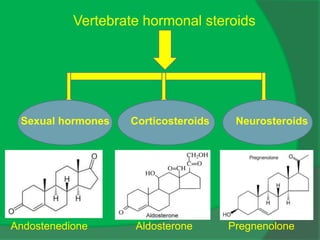
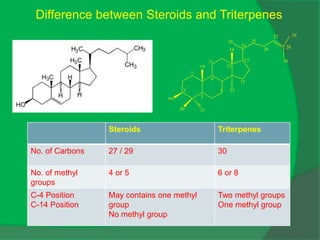
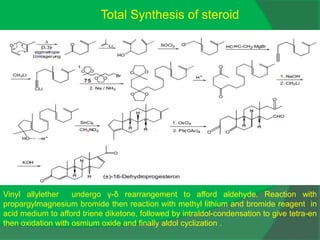
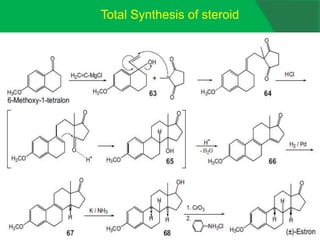
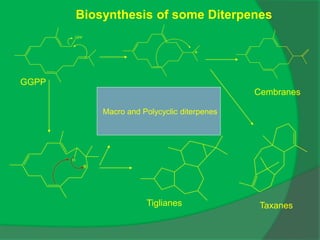
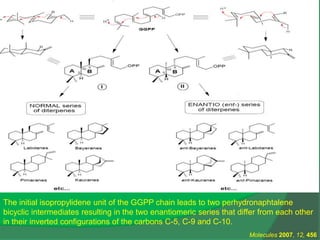
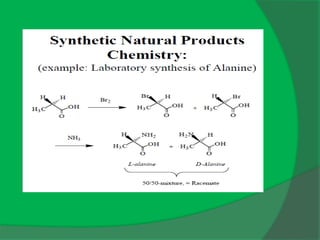
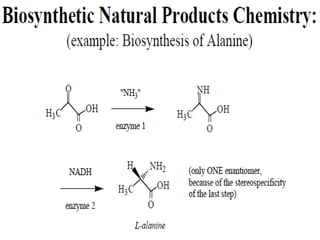
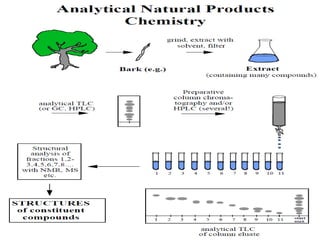
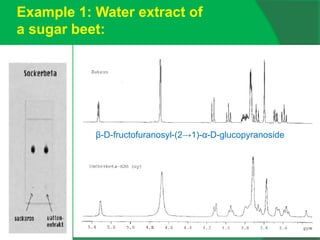
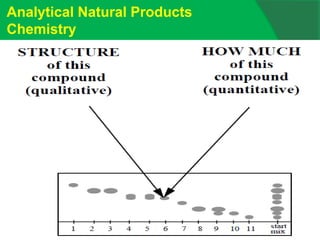
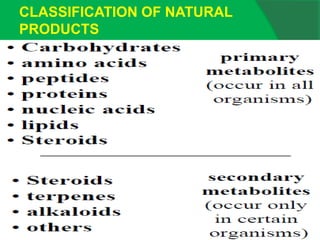
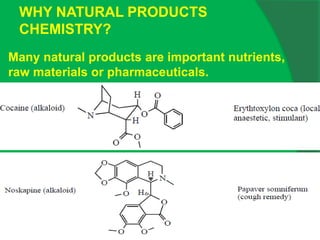
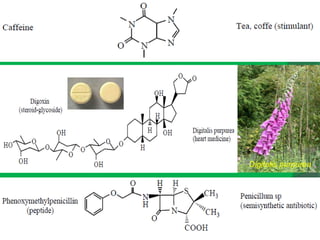
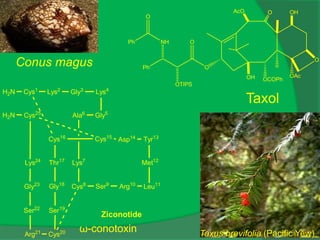
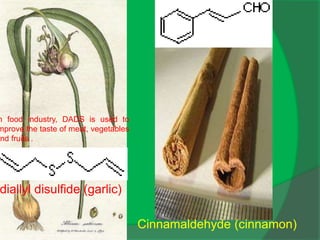
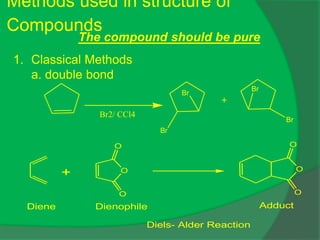
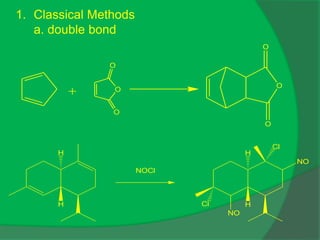
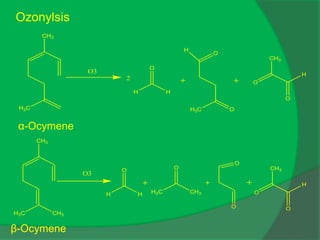
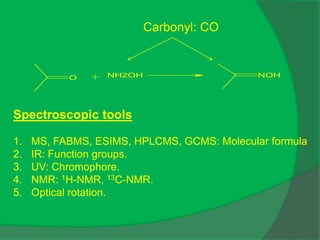
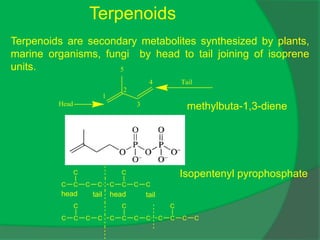
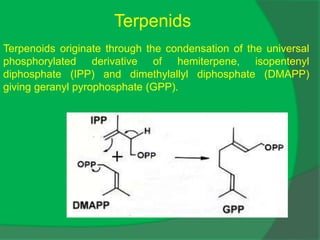
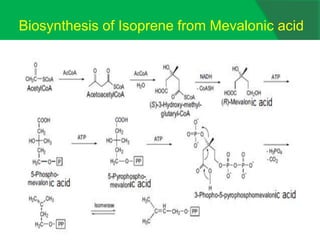
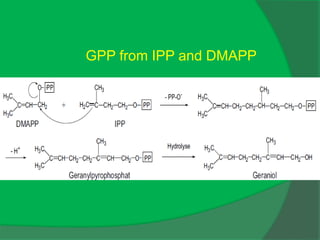
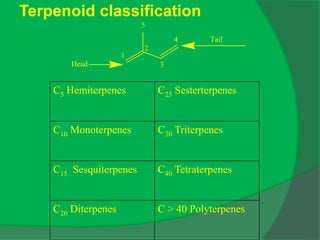
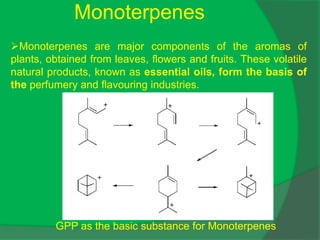
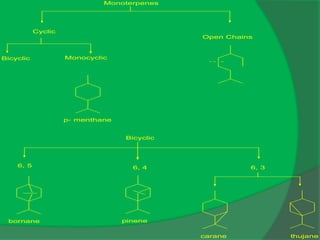
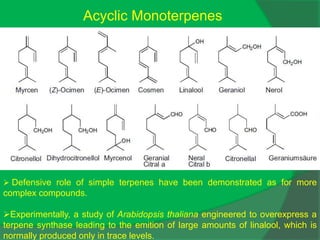
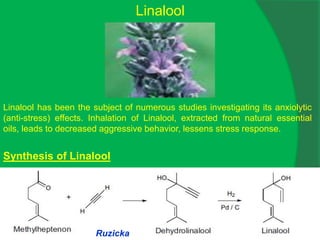
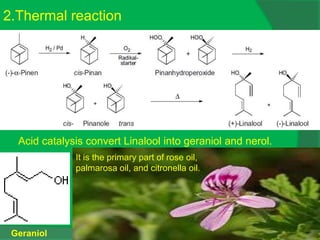
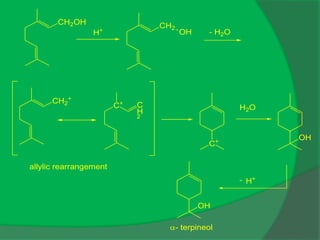
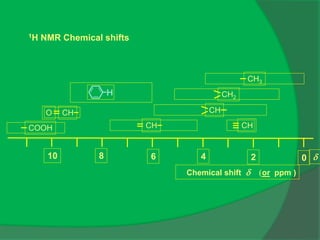
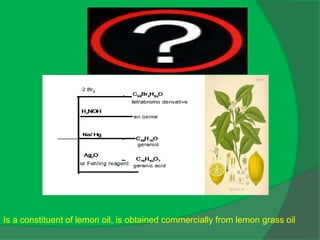
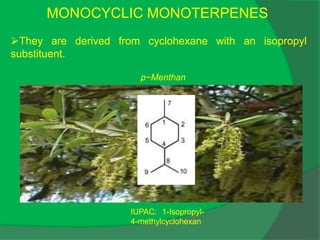
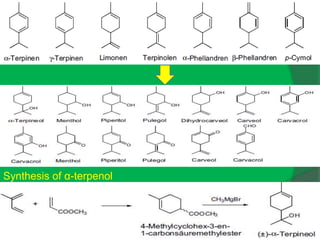
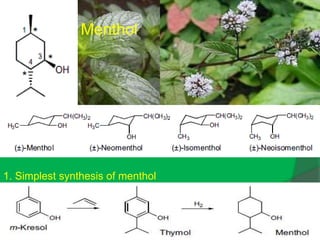
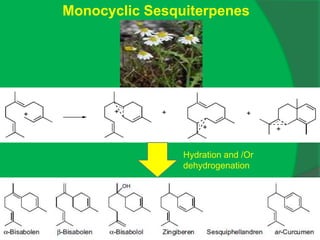
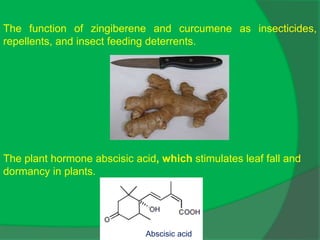
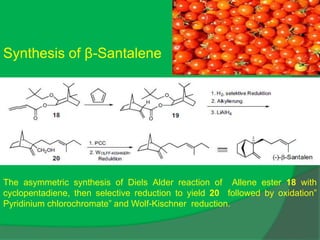
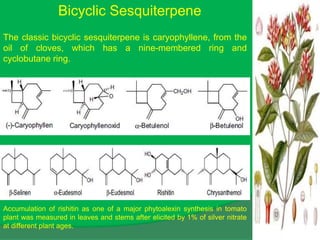
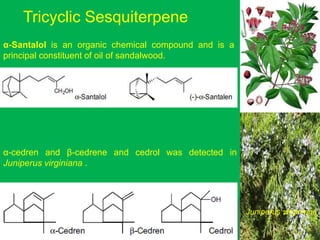
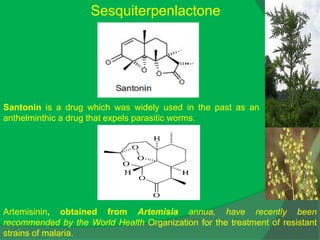
The document covers natural product chemistry, focusing on the importance of naturally occurring compounds, their classification into primary and secondary metabolites, and the chemical methods for their isolation and structure elucidation. It discusses the biological significance of secondary metabolites, including their roles in medicine and ecology, as well as the biosynthesis of various classes of natural products like terpenoids. Additionally, it elaborates on analytical techniques used in characterizing these compounds and mentions historical uses and the development of pharmaceuticals from natural substances.










![0
1
2
3
PPM
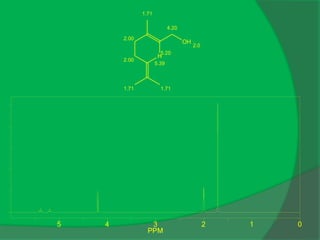
OH
1.52;1.27
1.52;1.27
1.50
3.16
1.67;1.42
1.61
1.82
1.01 1.01
2.0
1.06
C10 H20O, [α]D = -50
IR: 3333, 1048, 1028, 994](https://image.slidesharecdn.com/1-npcparti-2022-231029125214-059df7c1/85/1-NPC-Part-I-2022-ppt-52-320.jpg)
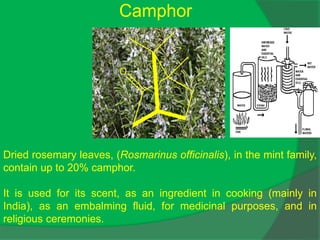
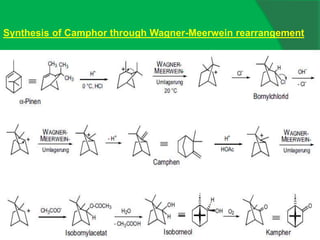
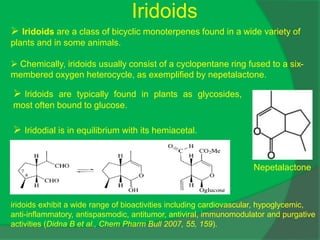
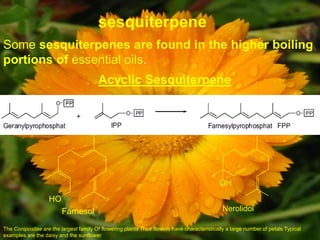
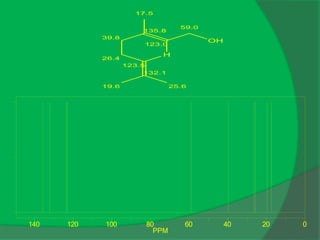
![IR (KBr): νmax = 3415, 2935, 2870, 1461, 1376, 1074, 1026.
EIMS: m/z (rel. int.) = 256 ([M]+, < 1), 241 ([M-CH3]+, 3.5), 223
([241- H2O]+, 3.8), 205 ([241D2H2O]+, 2.1).
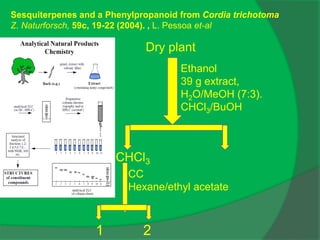
Sesquiterpenes and a Phenylpropanoid from Cordia trichotoma
Z. Naturforsch, 59c, 19-22 (2004).](https://image.slidesharecdn.com/1-npcparti-2022-231029125214-059df7c1/85/1-NPC-Part-I-2022-ppt-66-320.jpg)