Embed presentation
Download to read offline

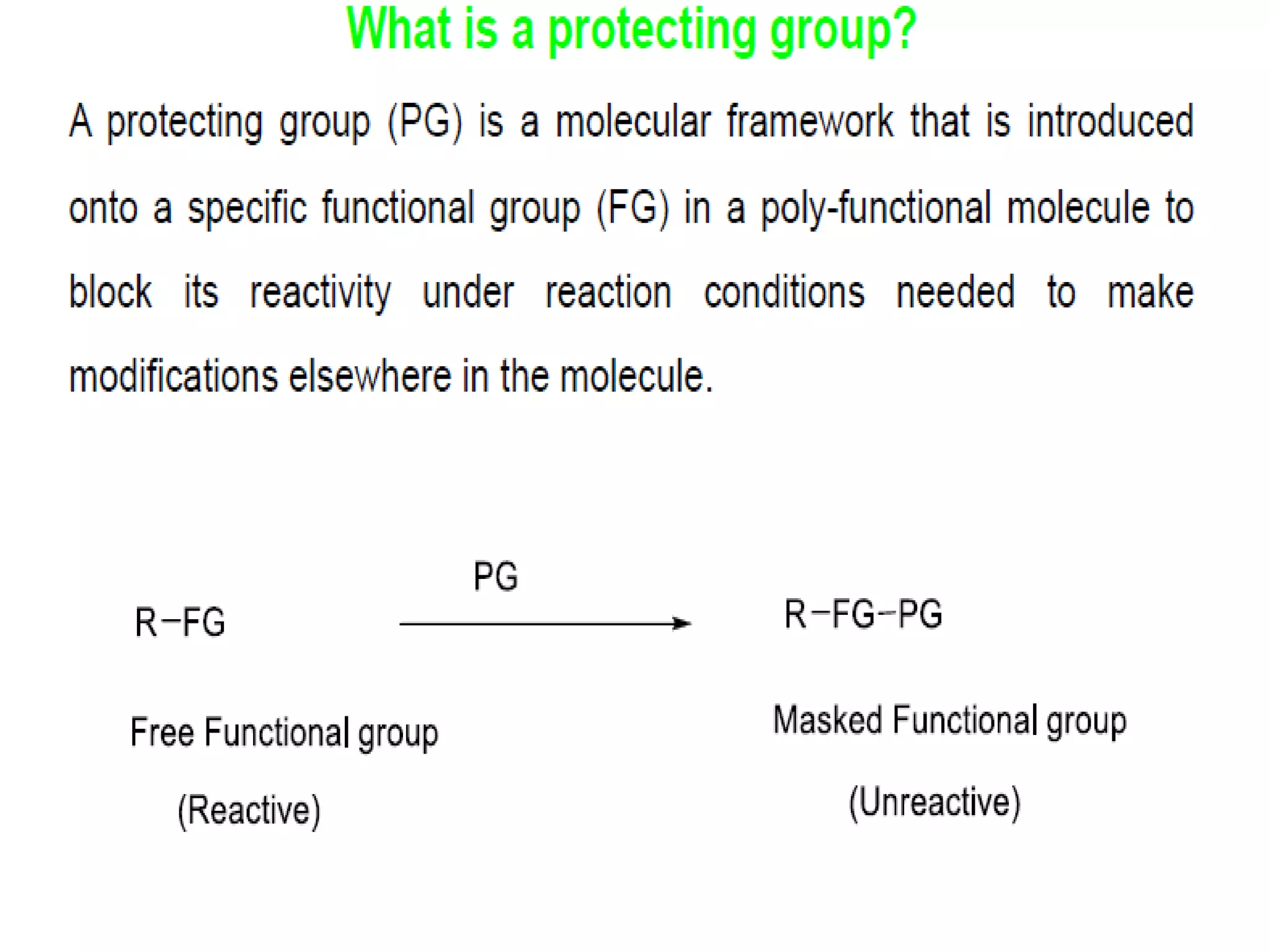

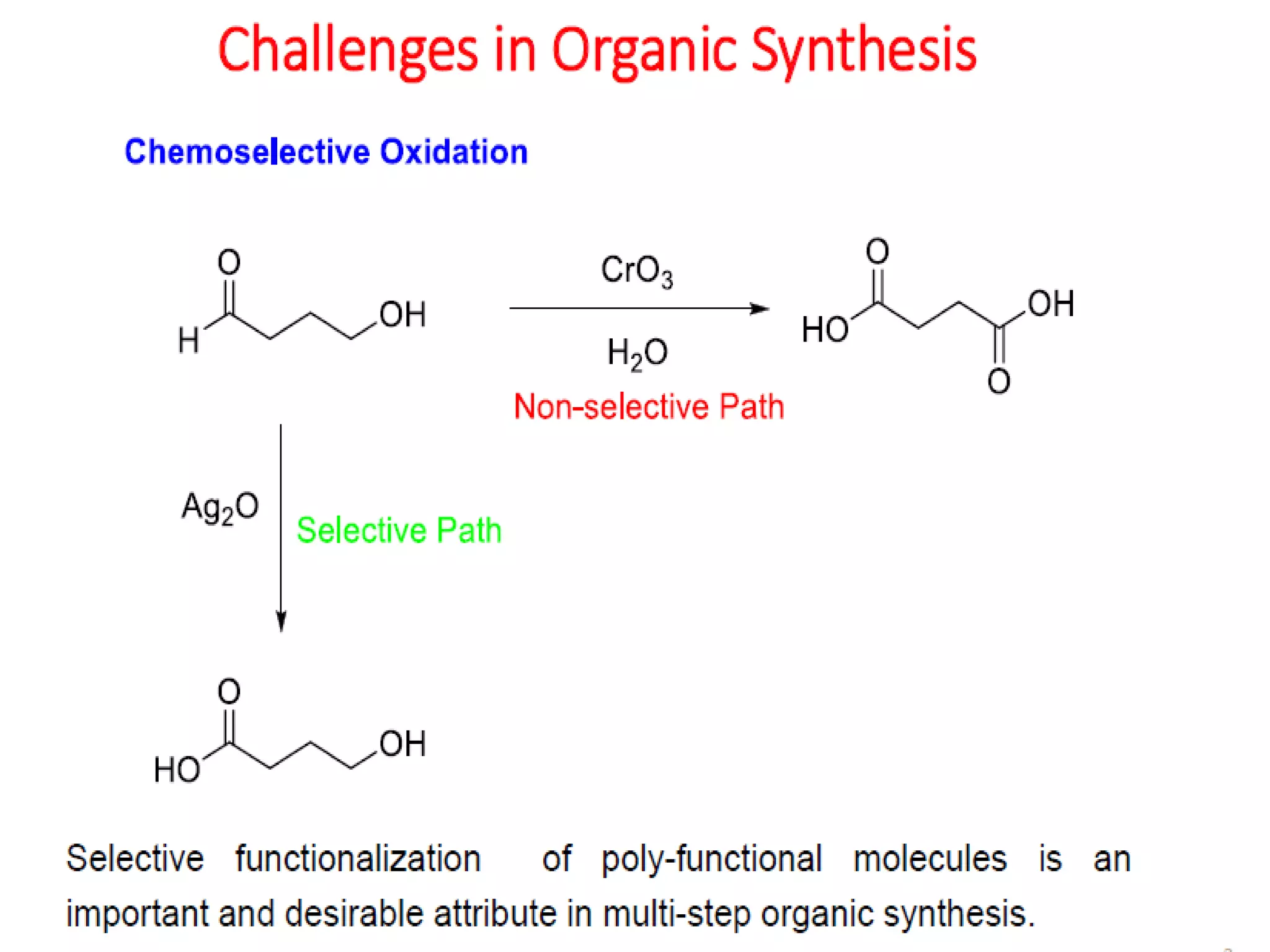
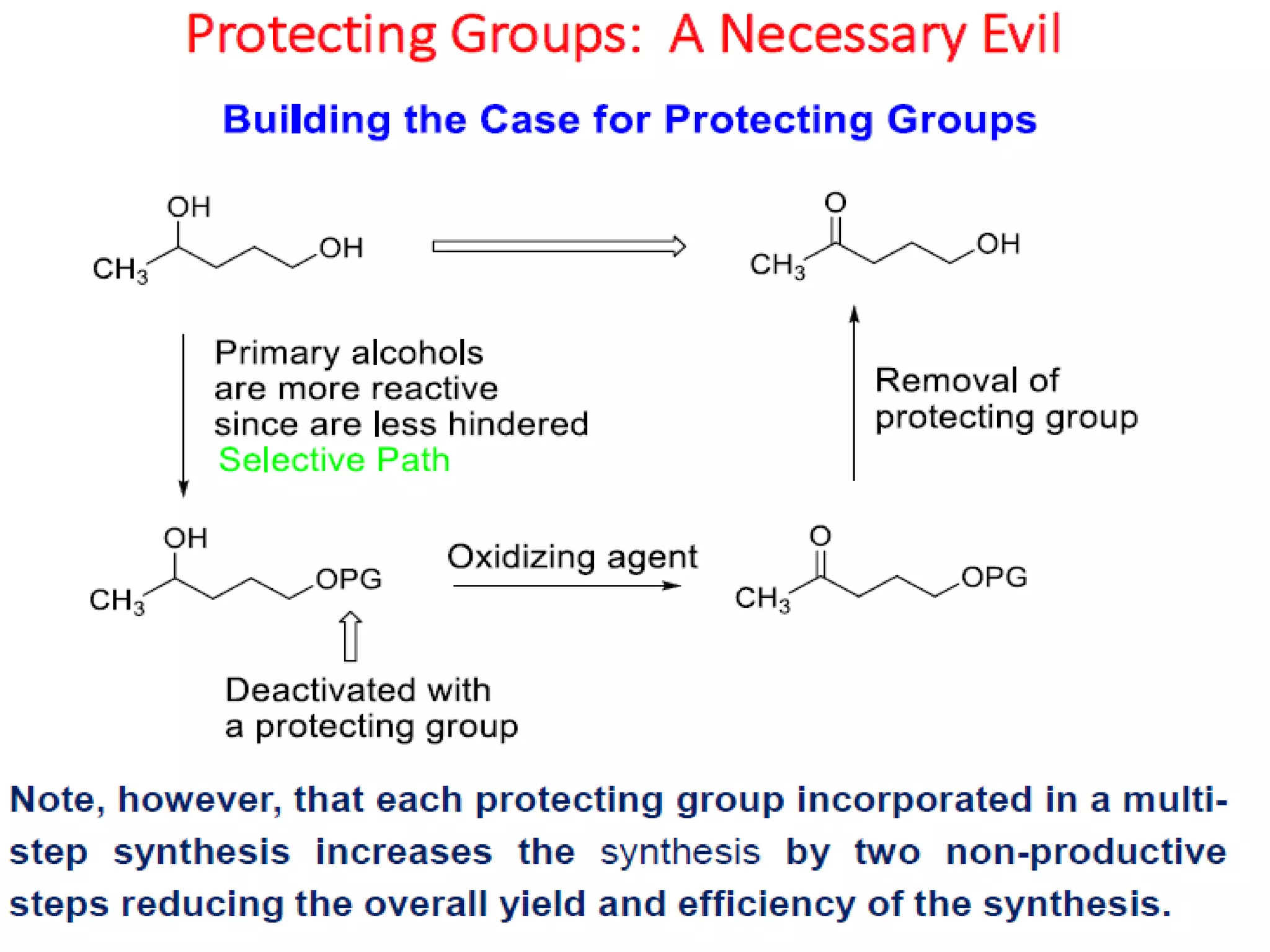

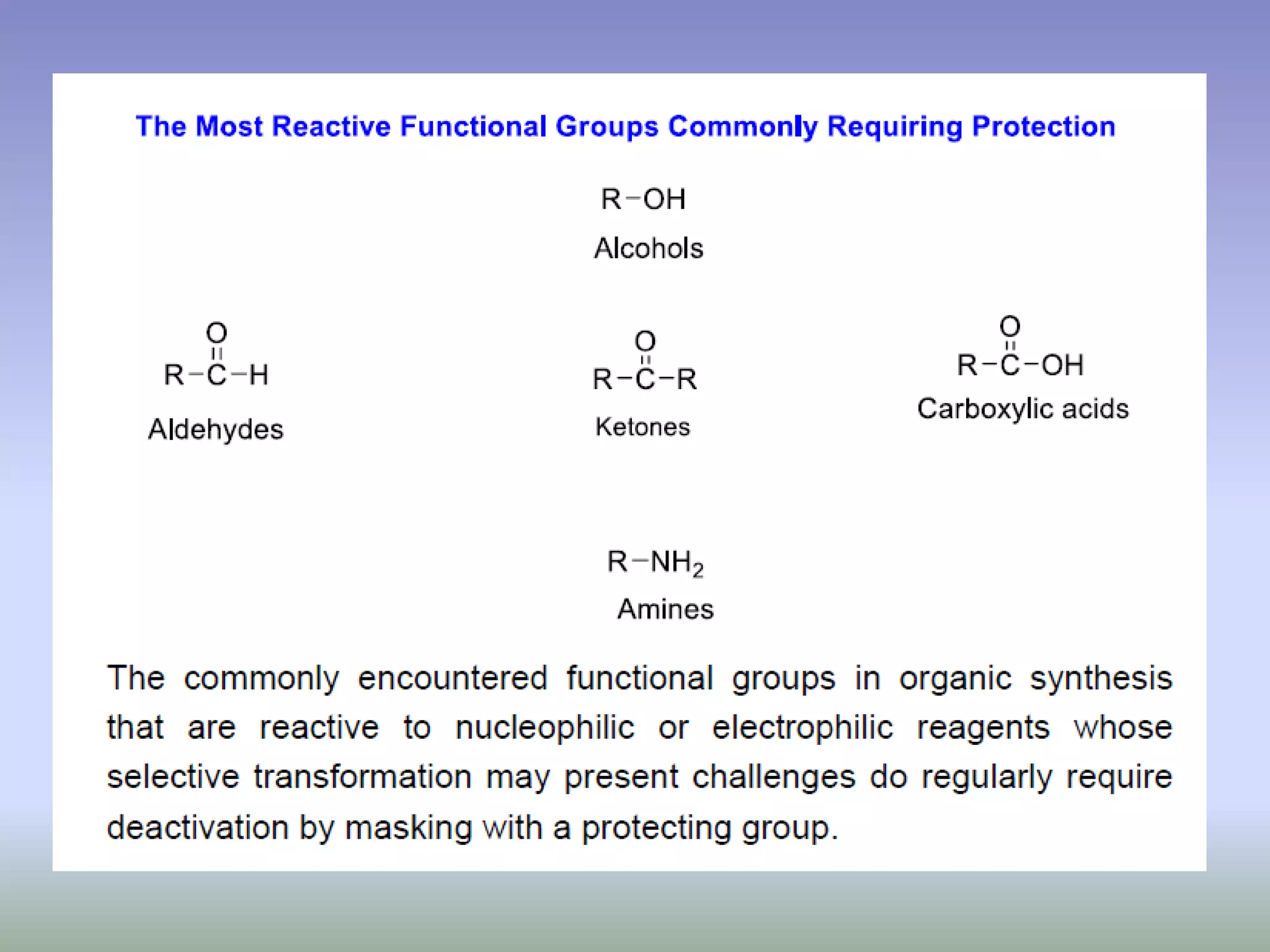

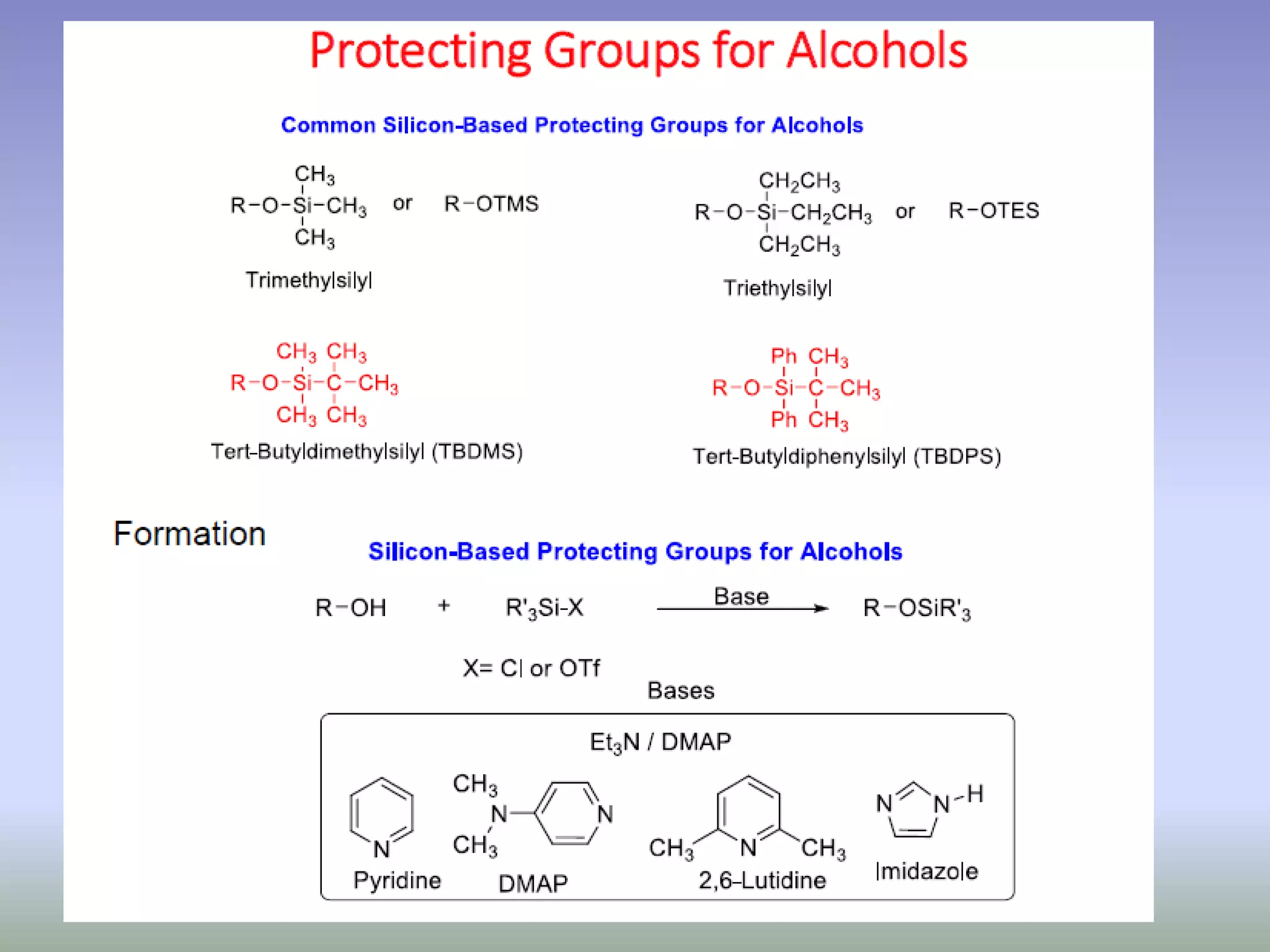
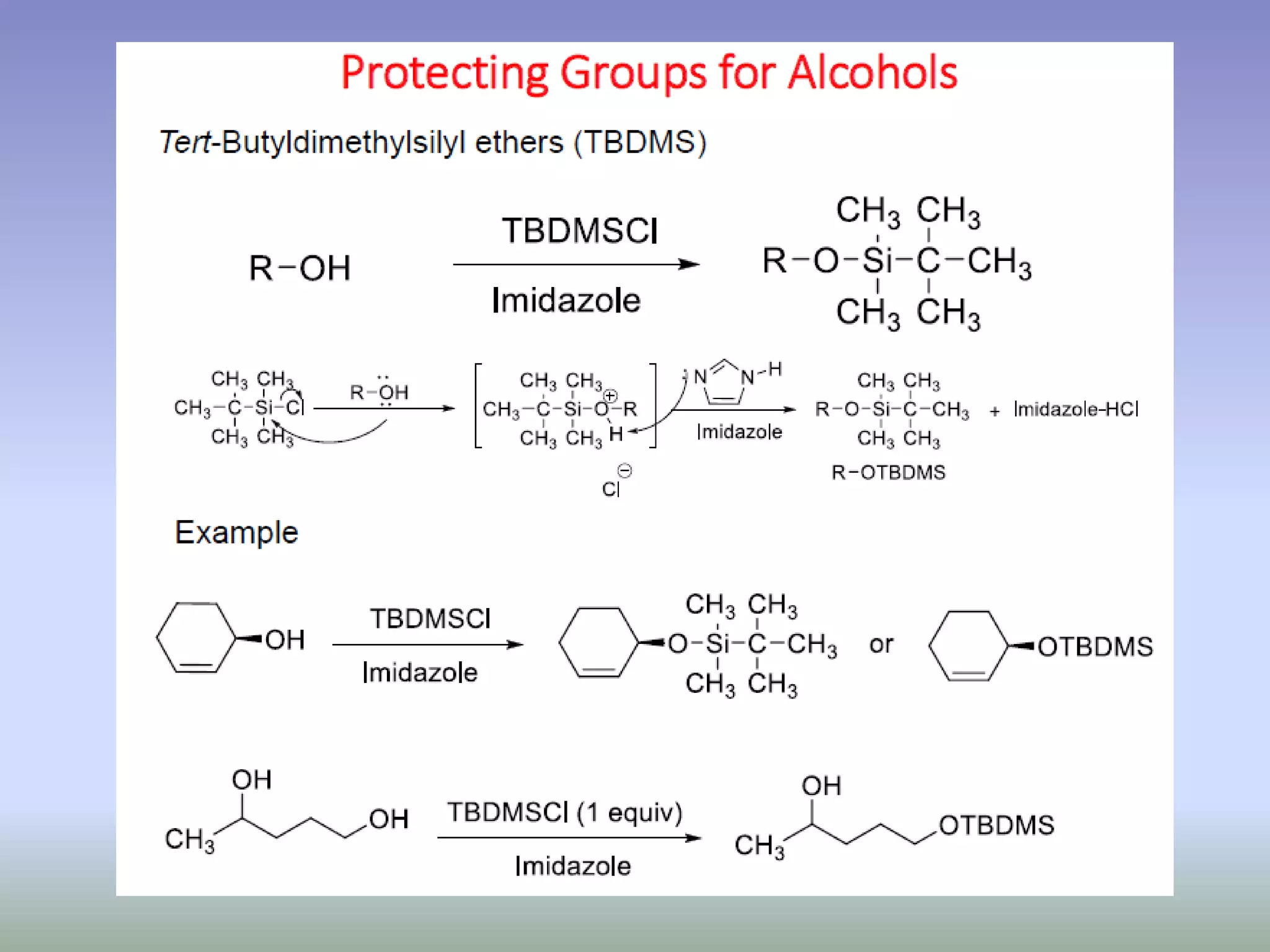
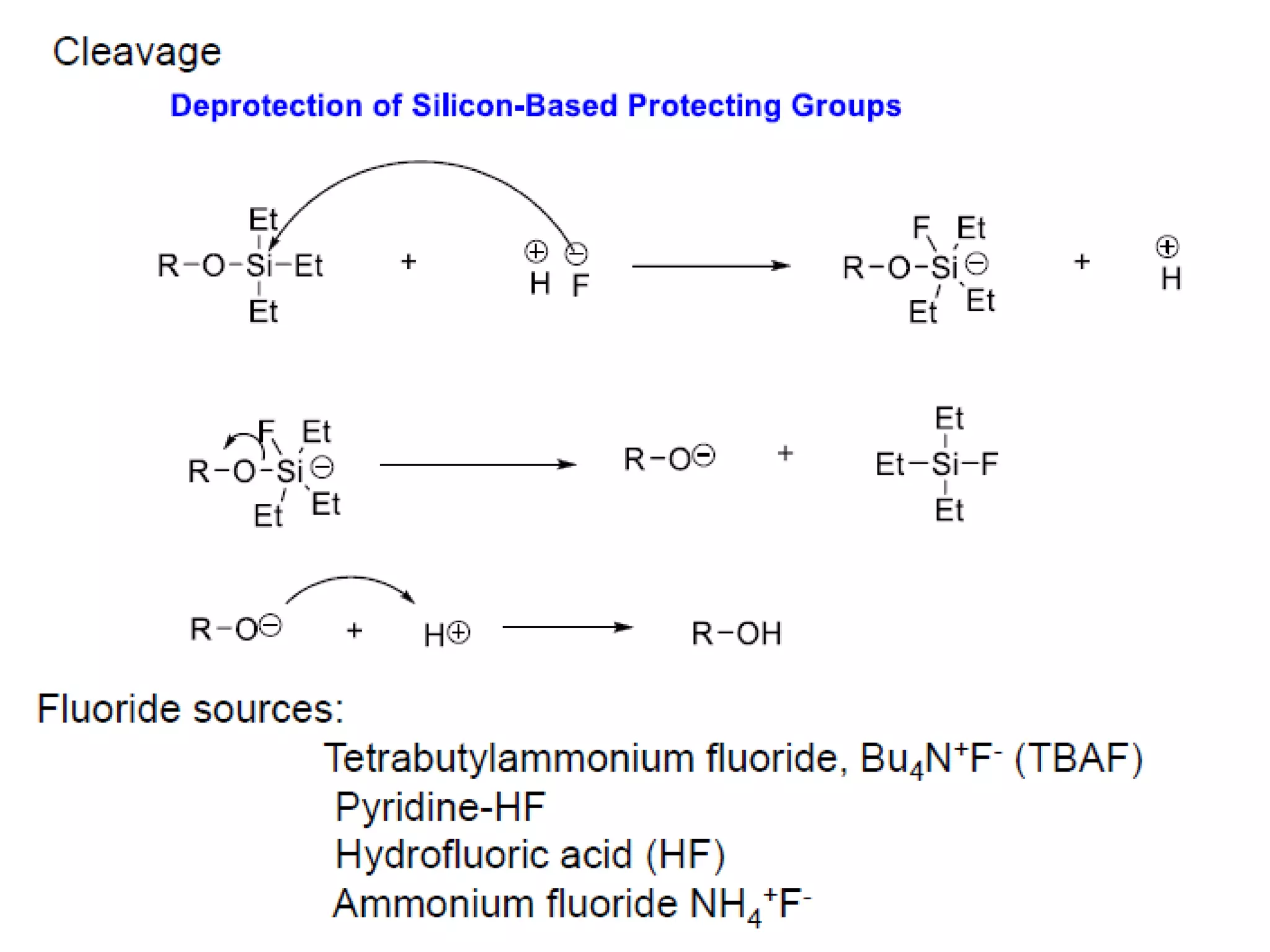
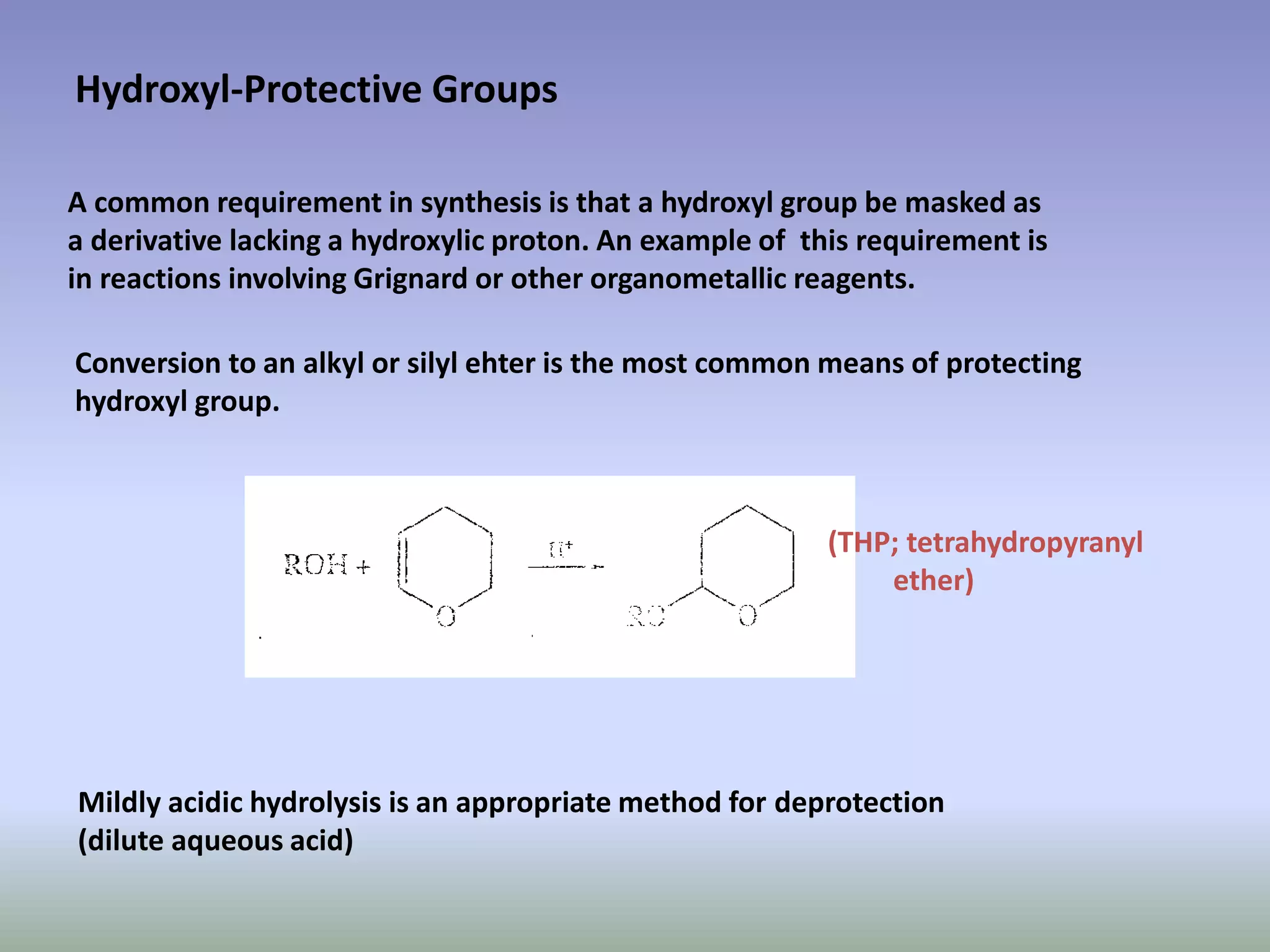
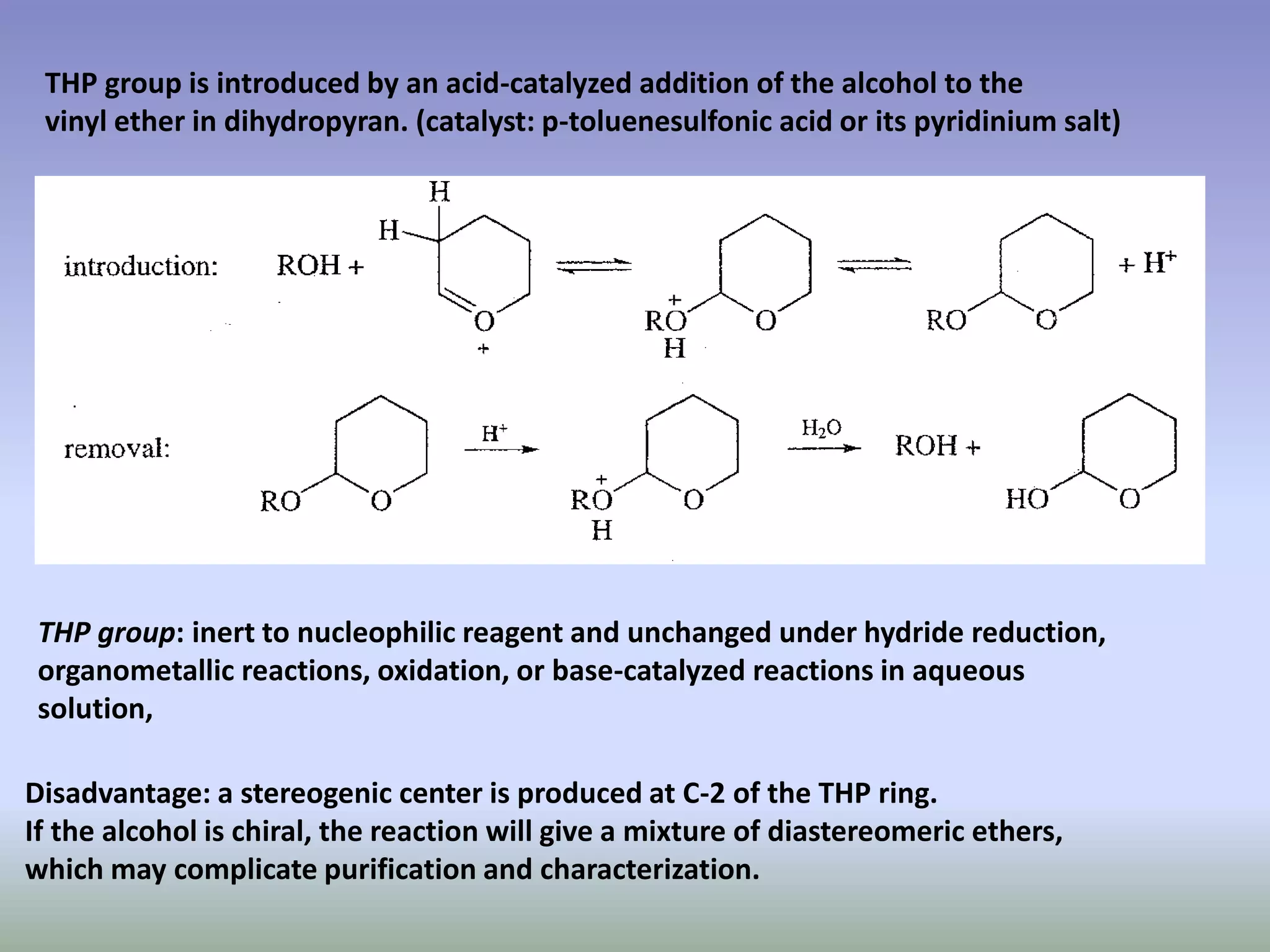
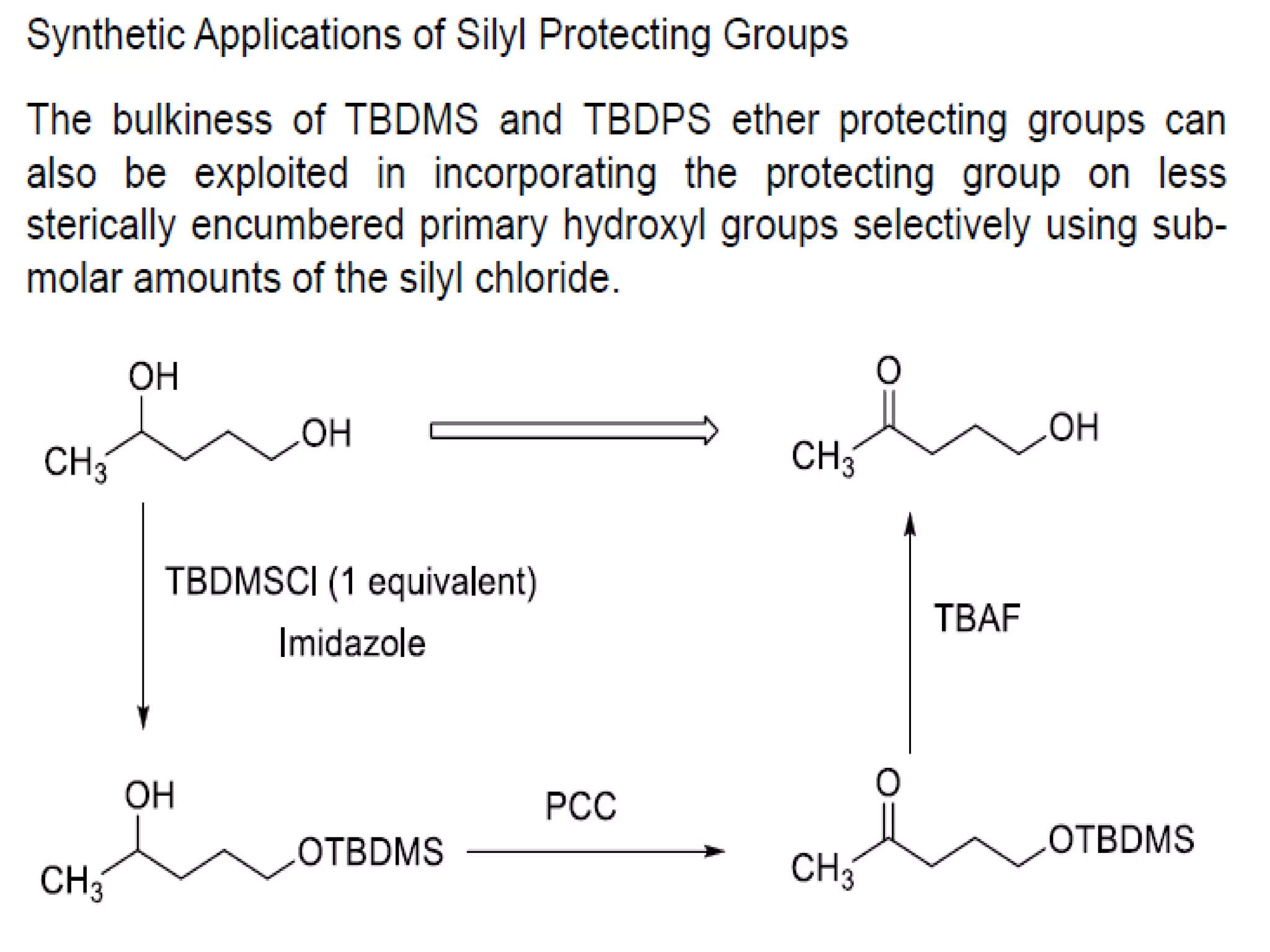
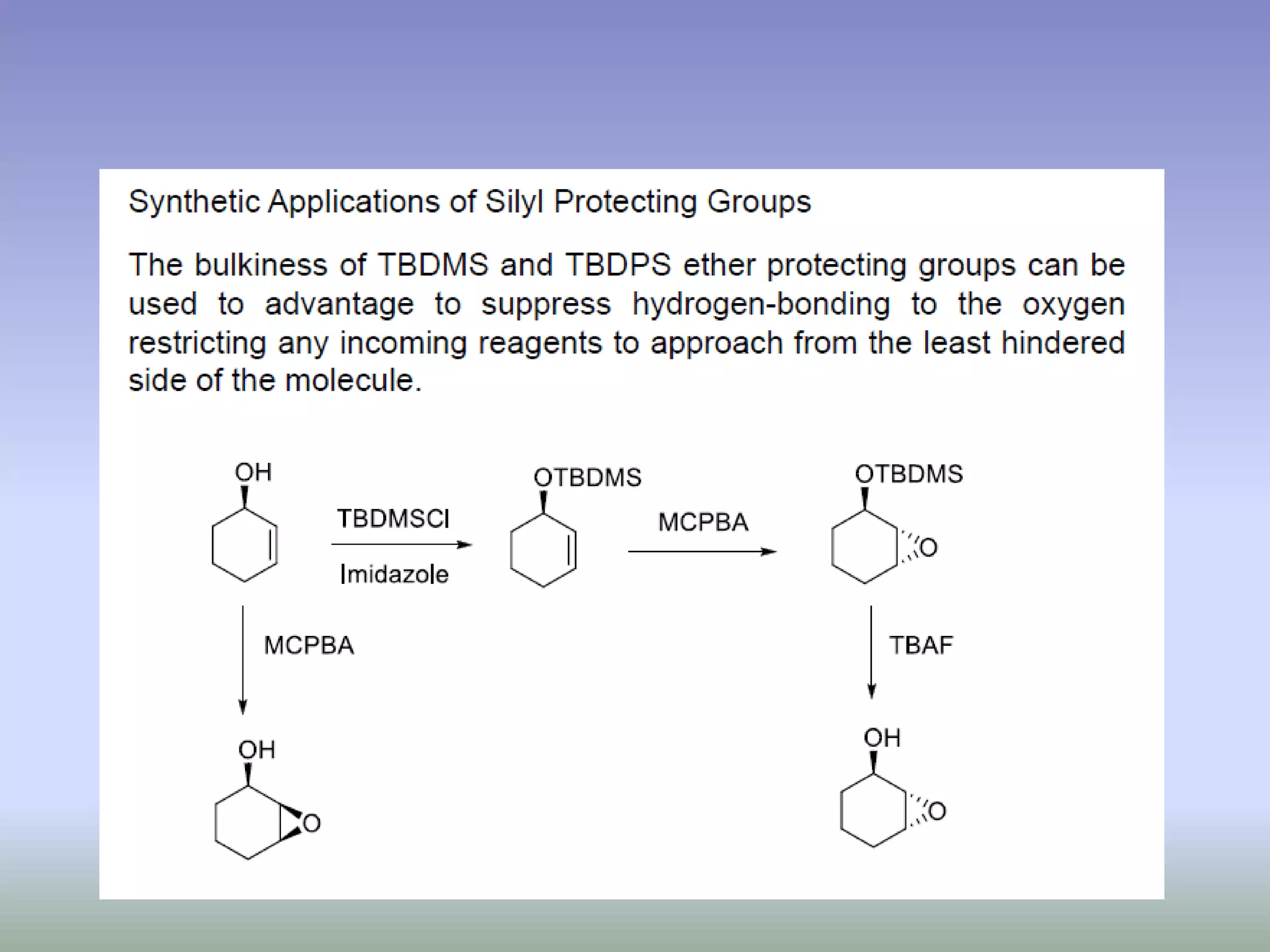
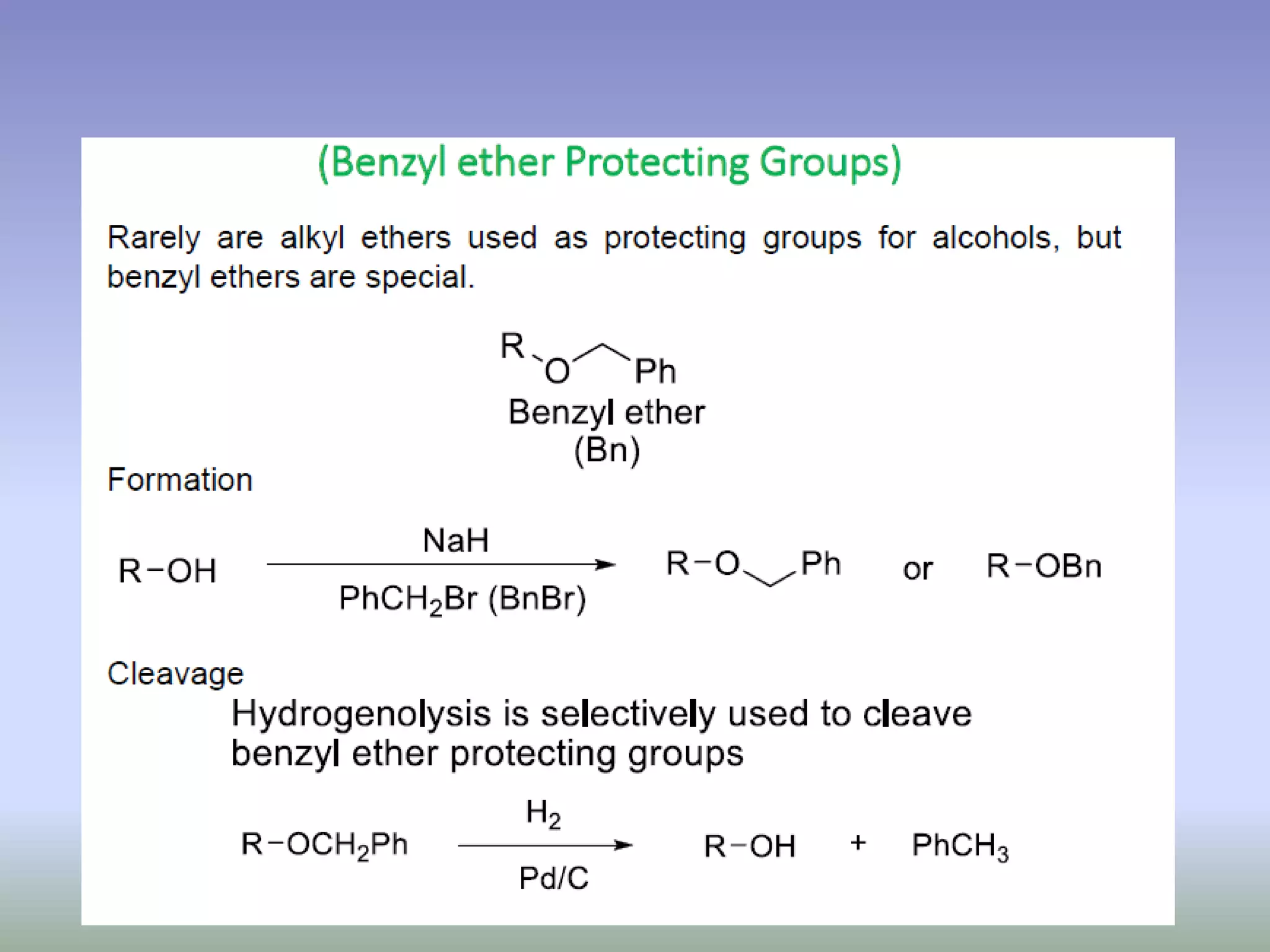
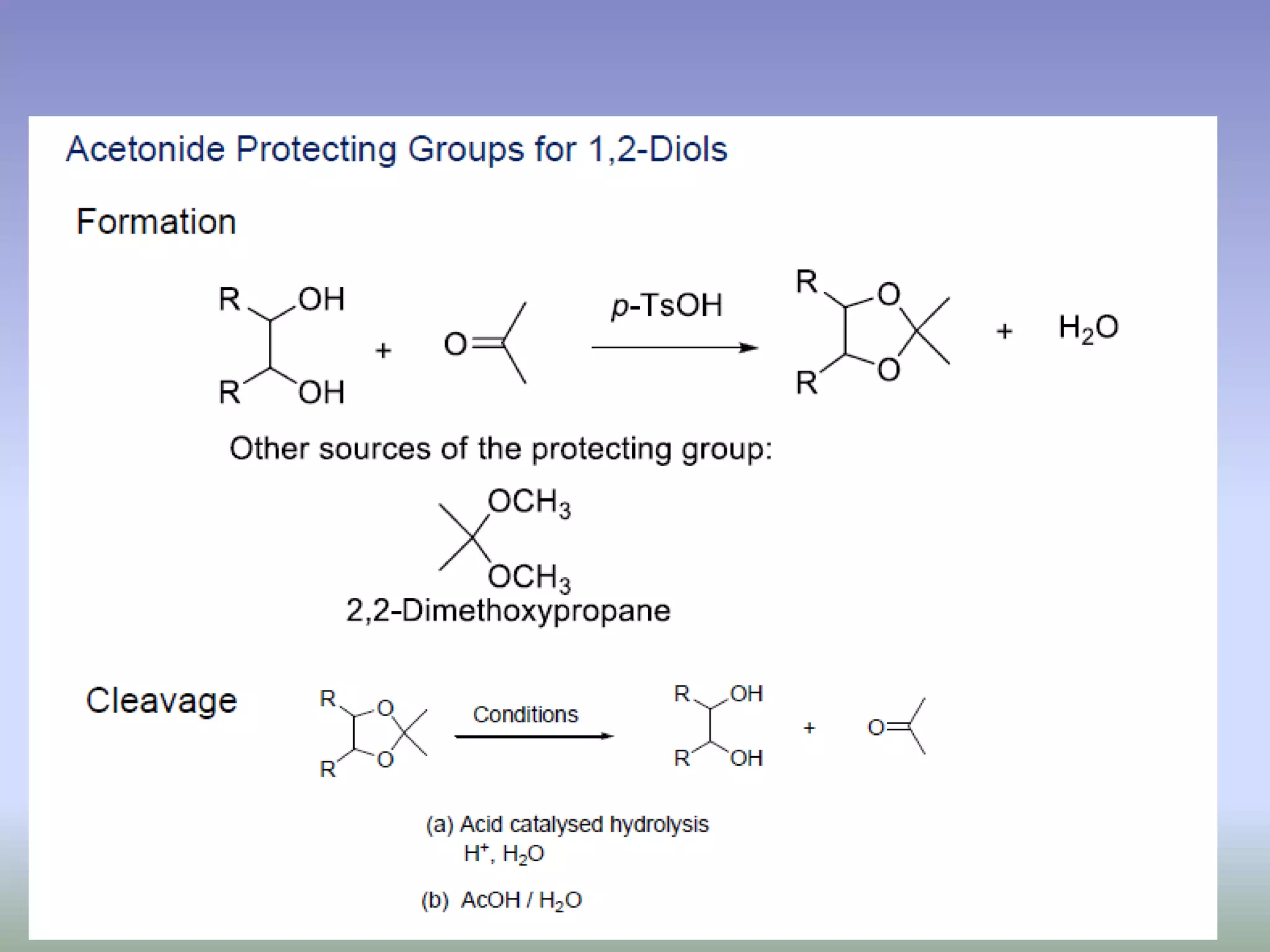
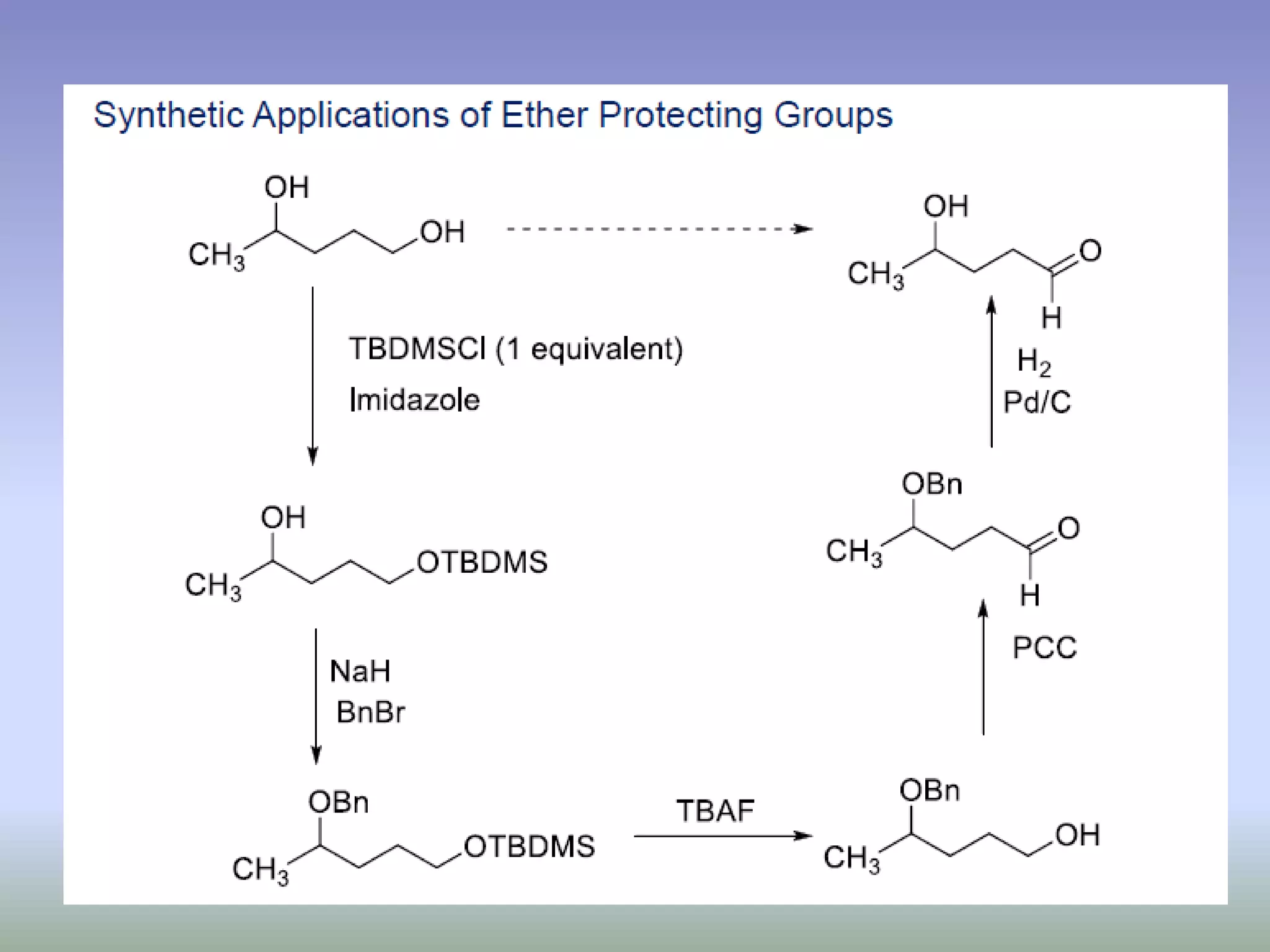
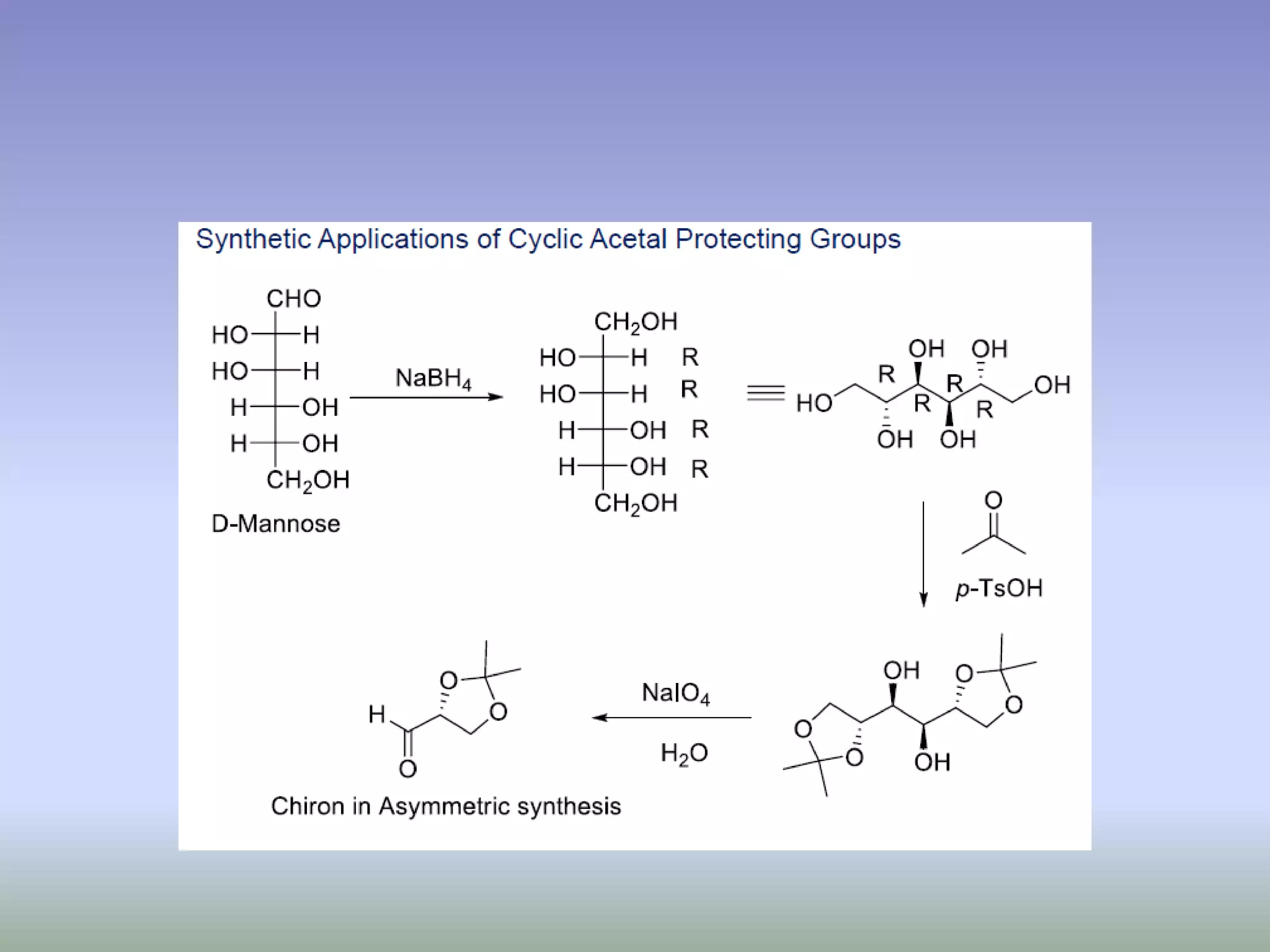
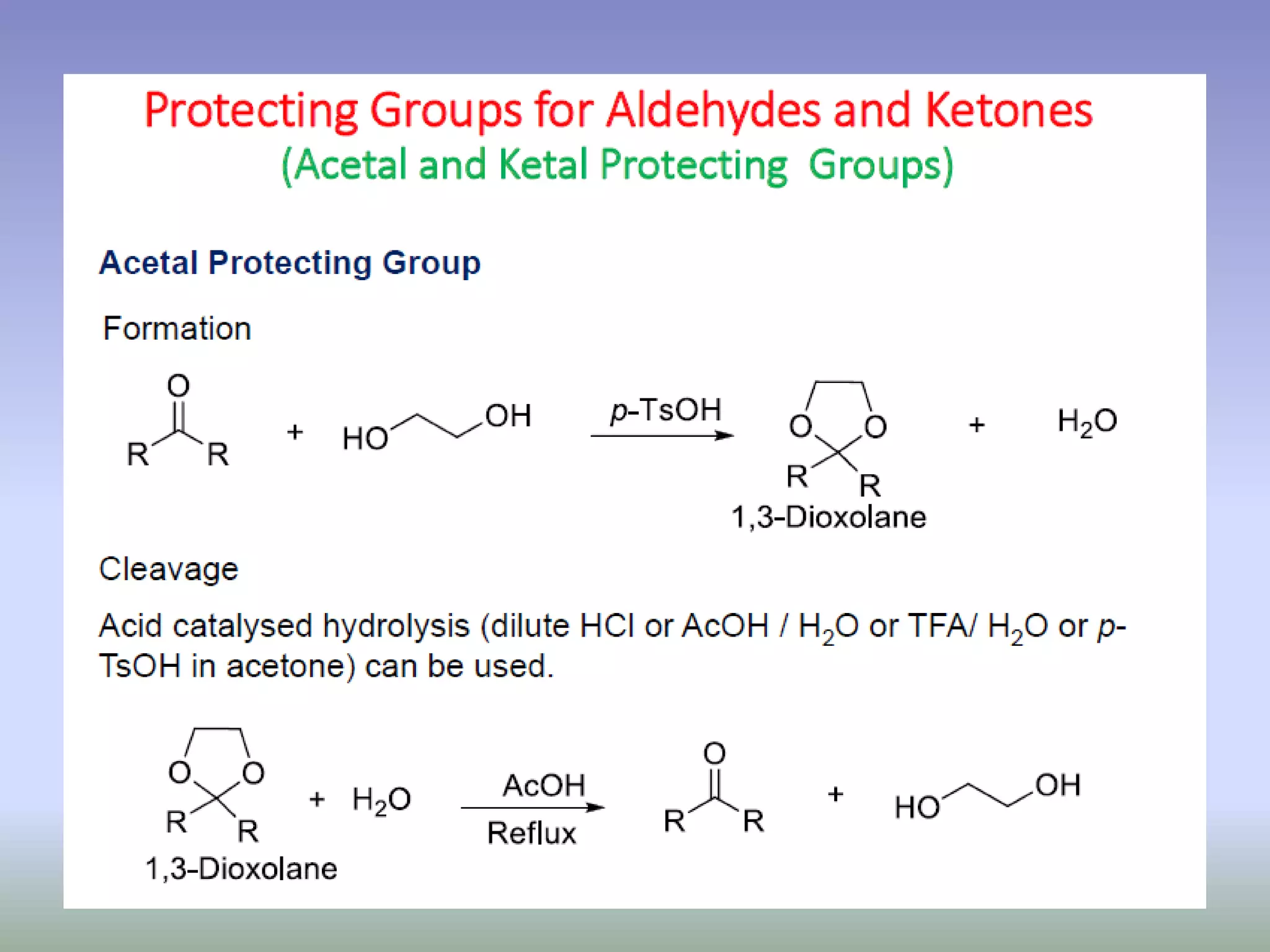
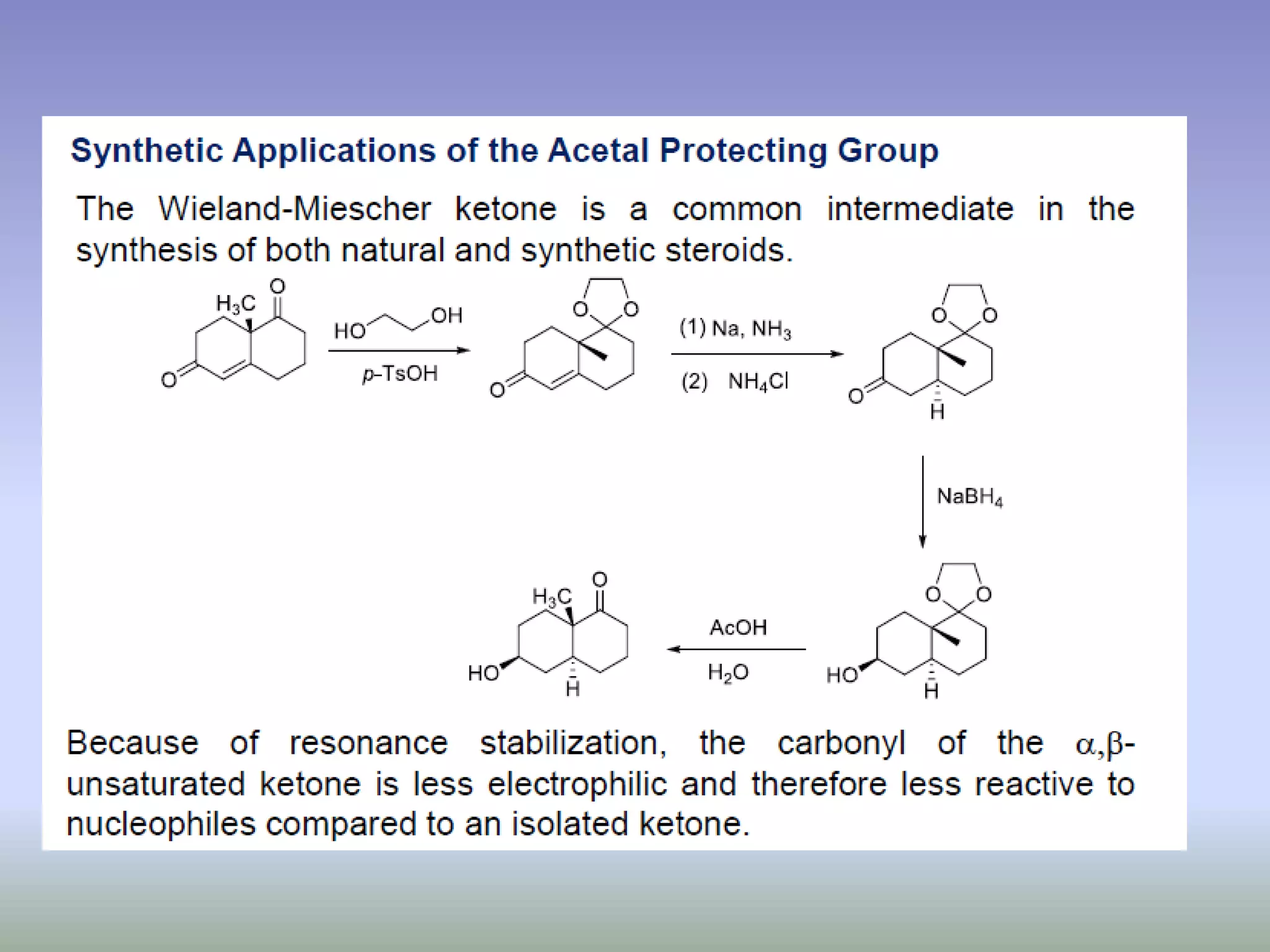
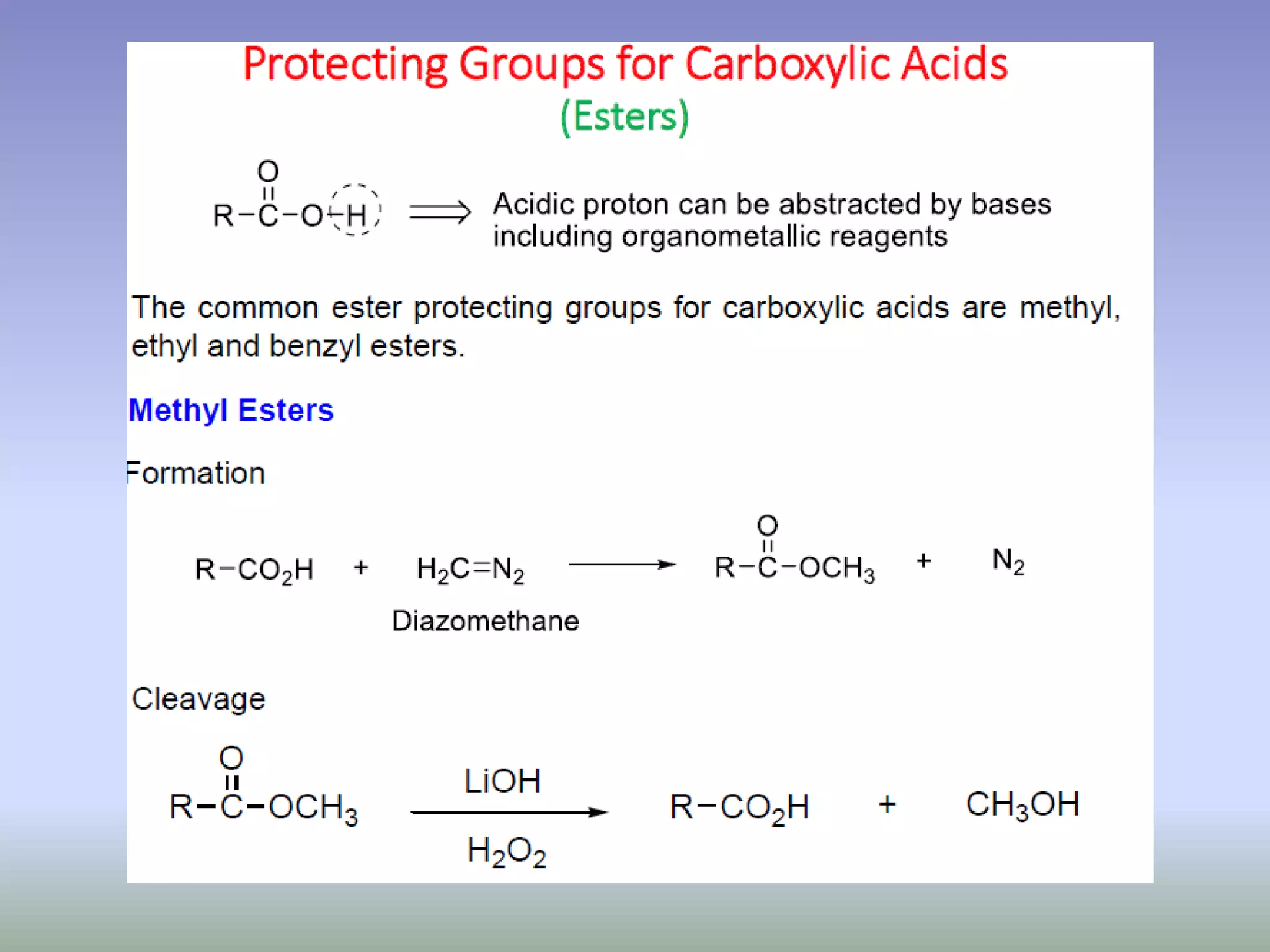
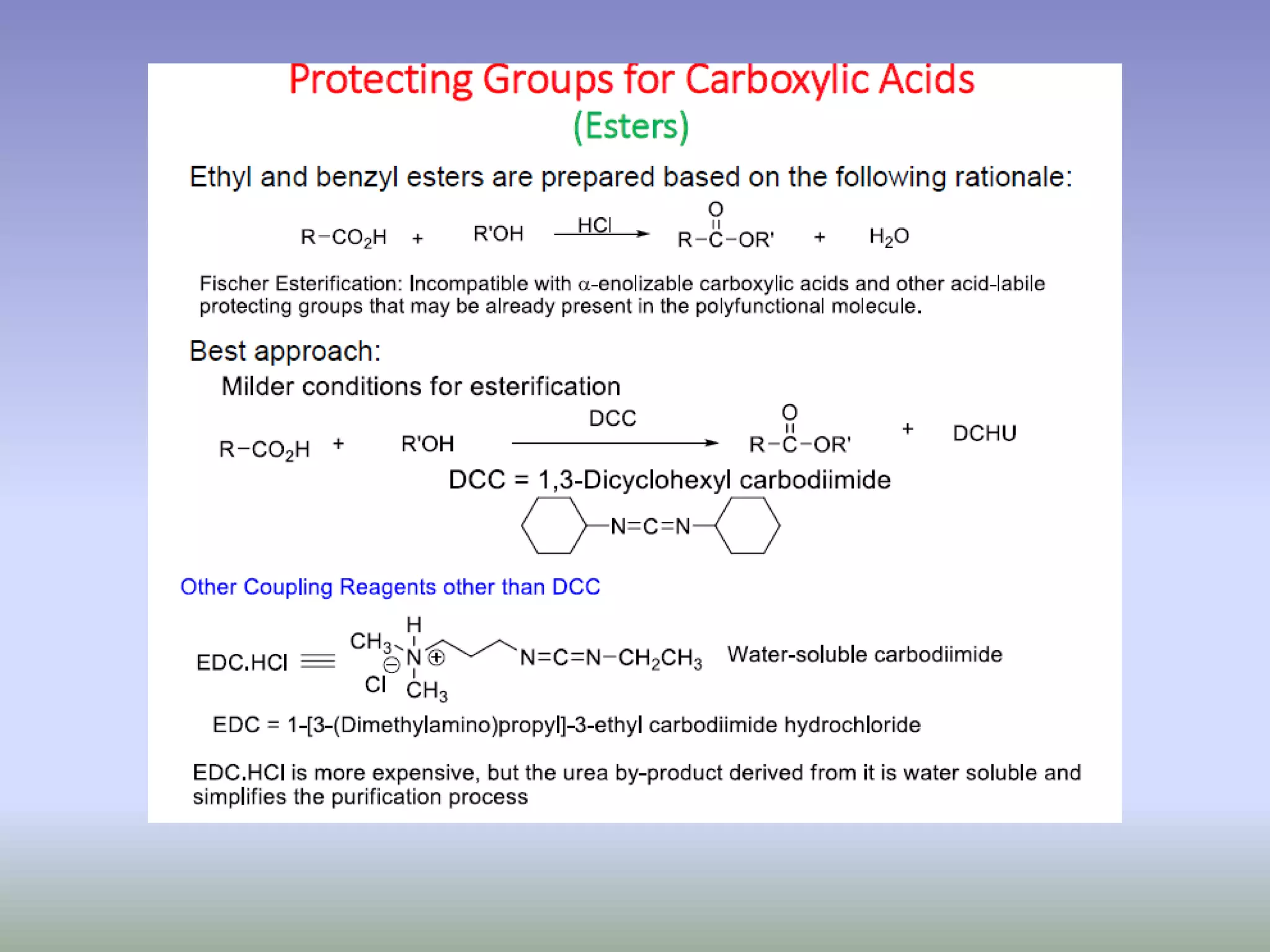
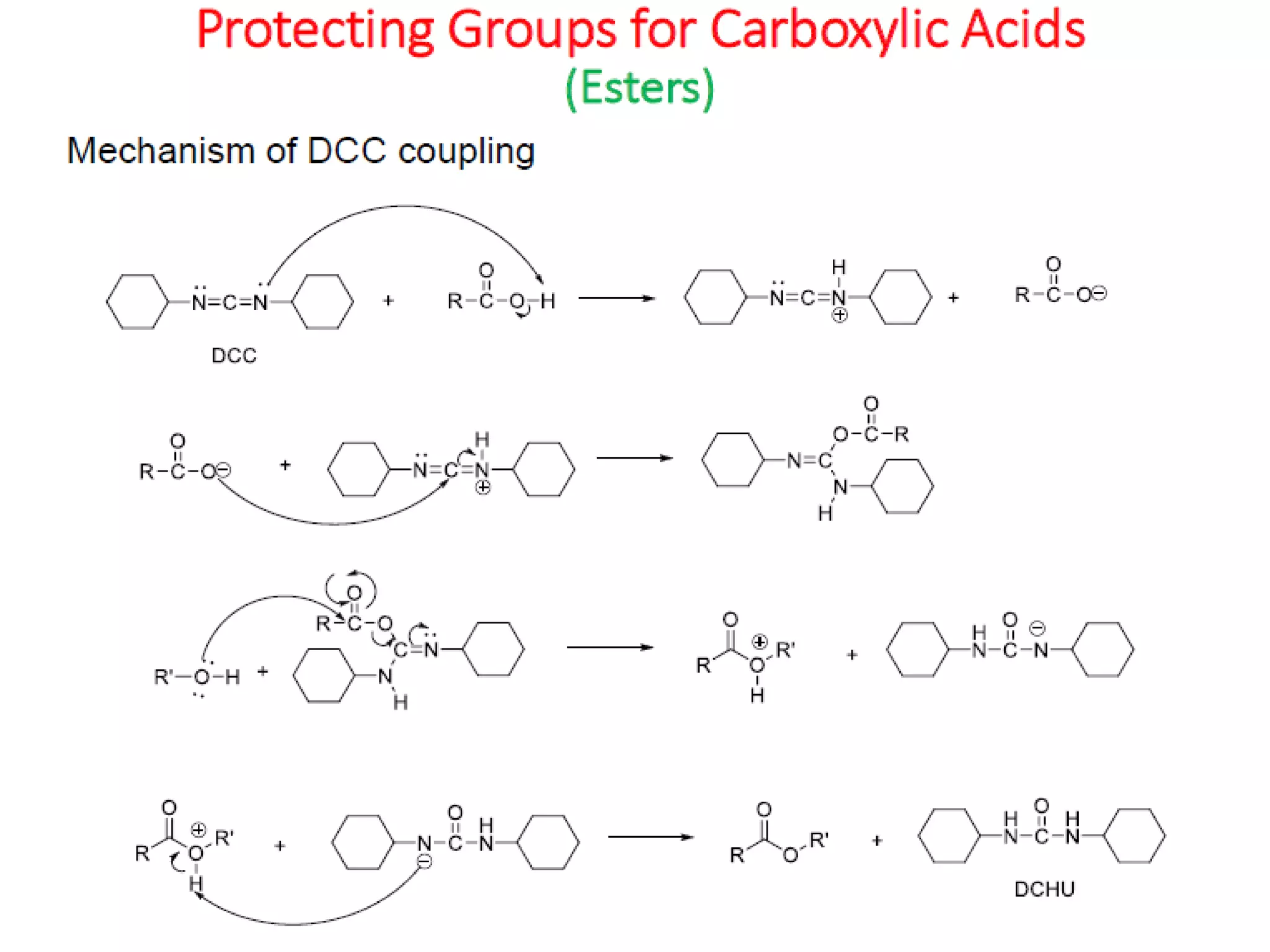
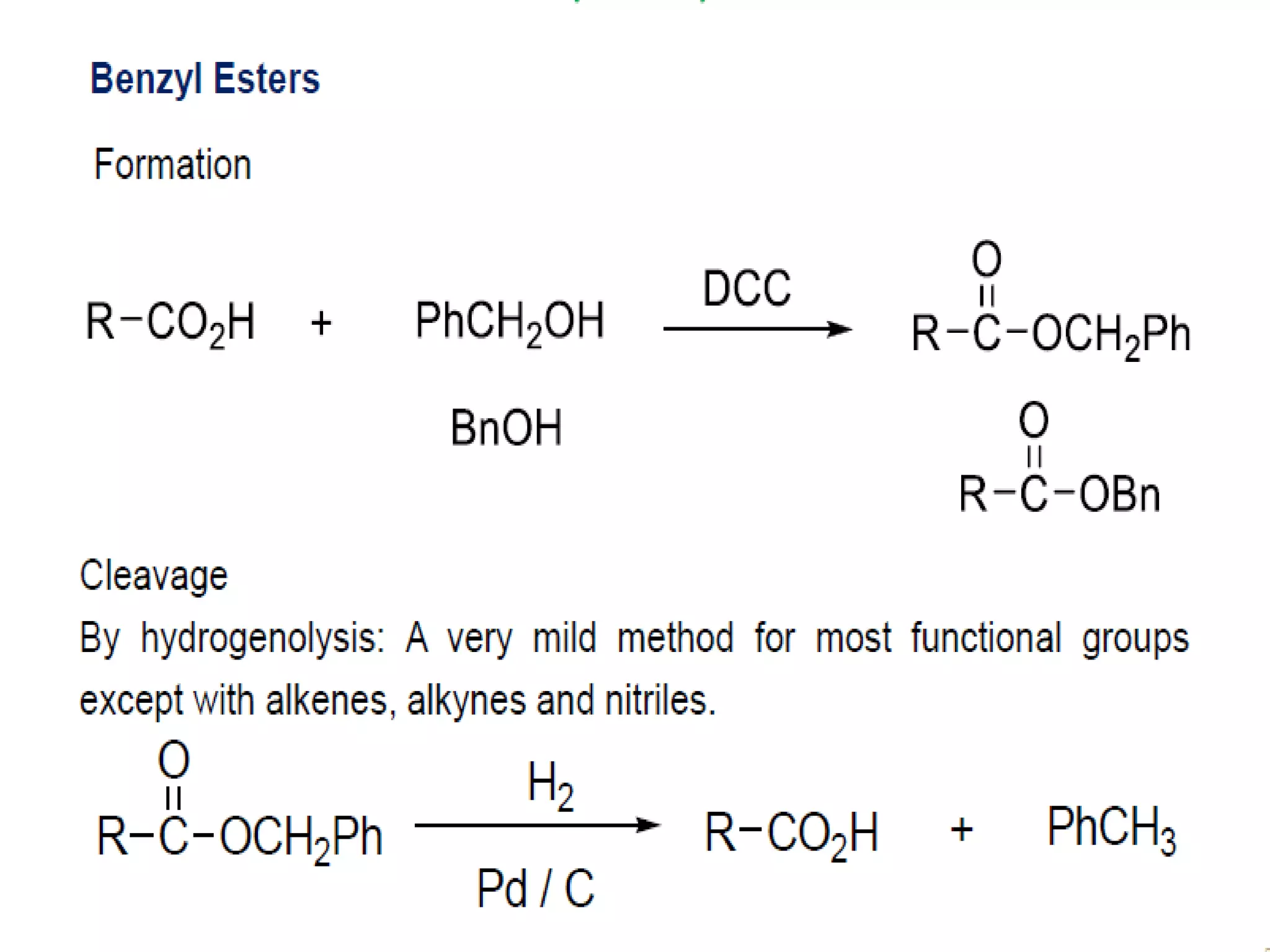
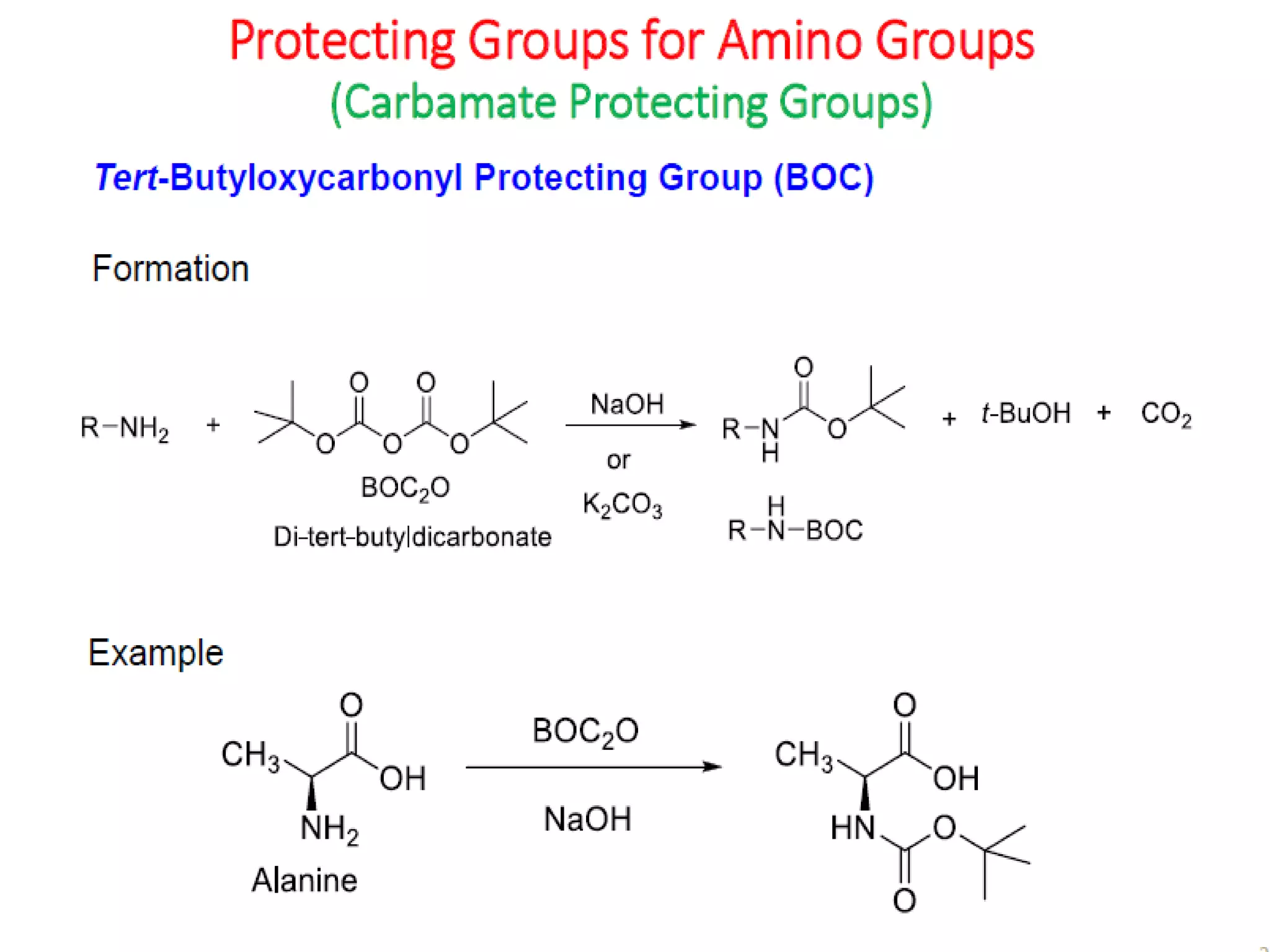
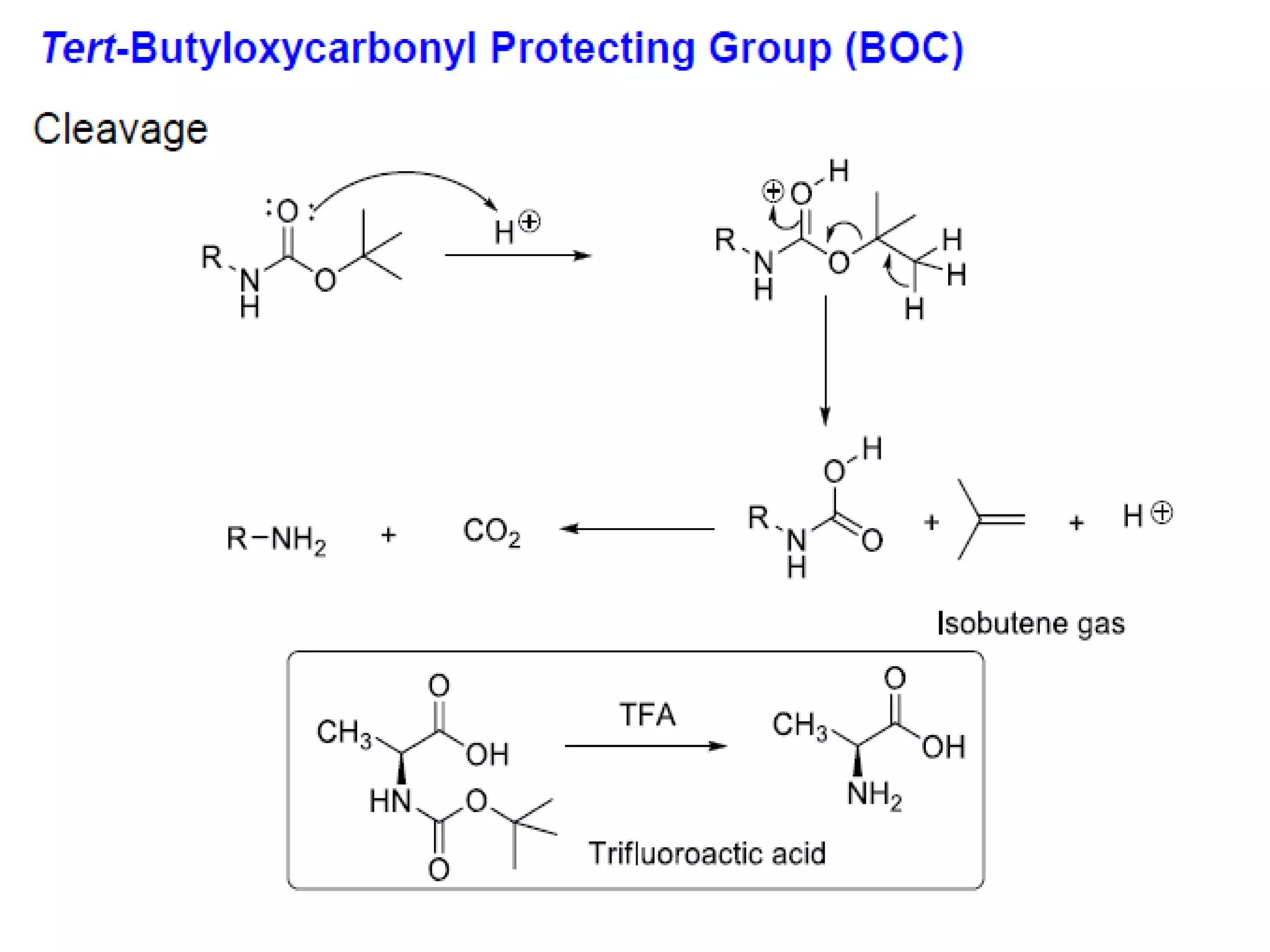
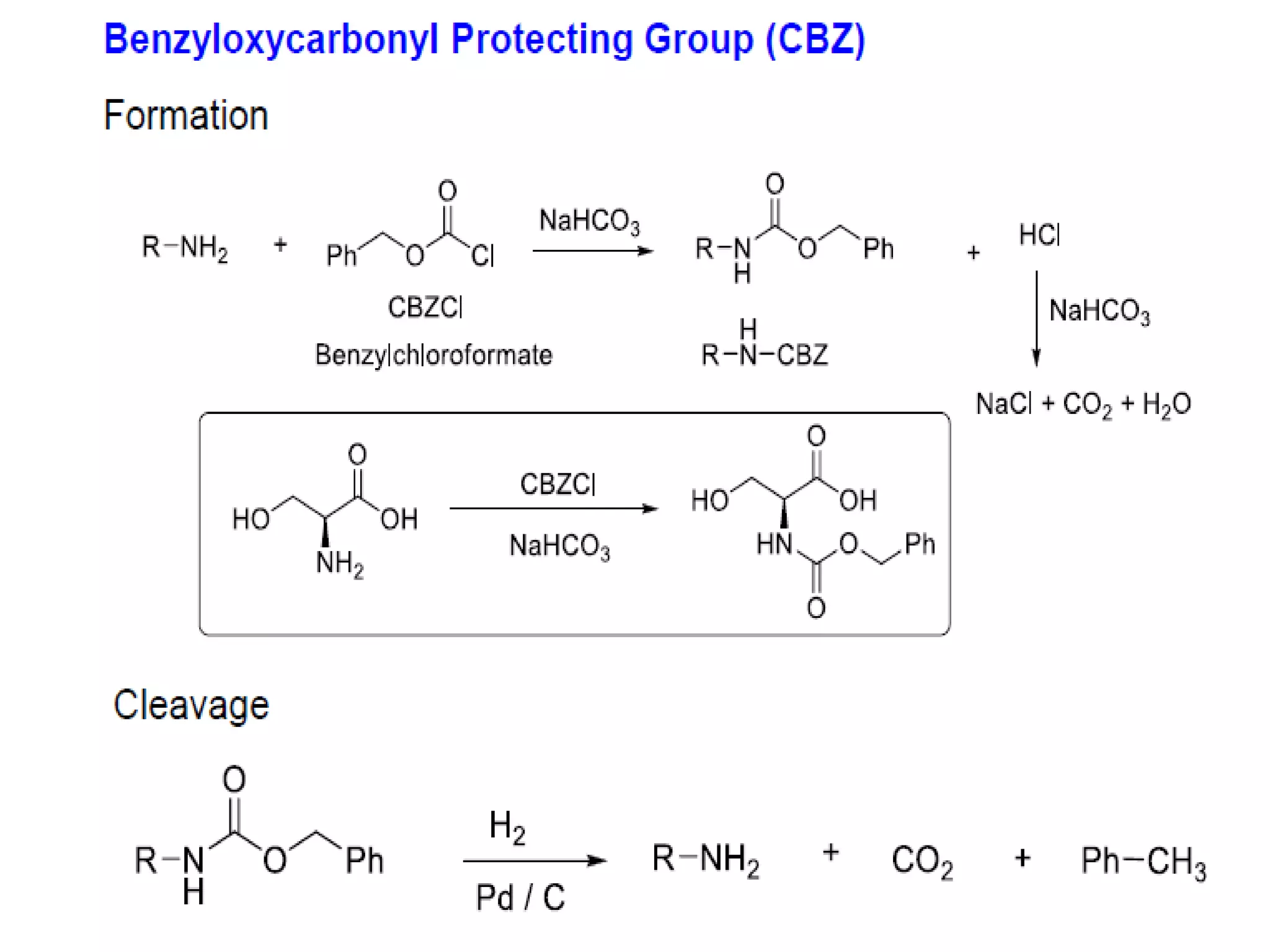


The document discusses the concept of protection and deprotection in chemical reactions, emphasizing the need to temporarily block reactive sites in multifunctional compounds to achieve selectivity. It describes requirements for a protecting group, noting the use of hydroxyl-protective groups like the tetrahydropyranyl (thp) ether, which can be introduced via acid-catalyzed addition. The text highlights the method of deprotection through mildly acidic hydrolysis and mentions potential complications in using chiral alcohols.




























