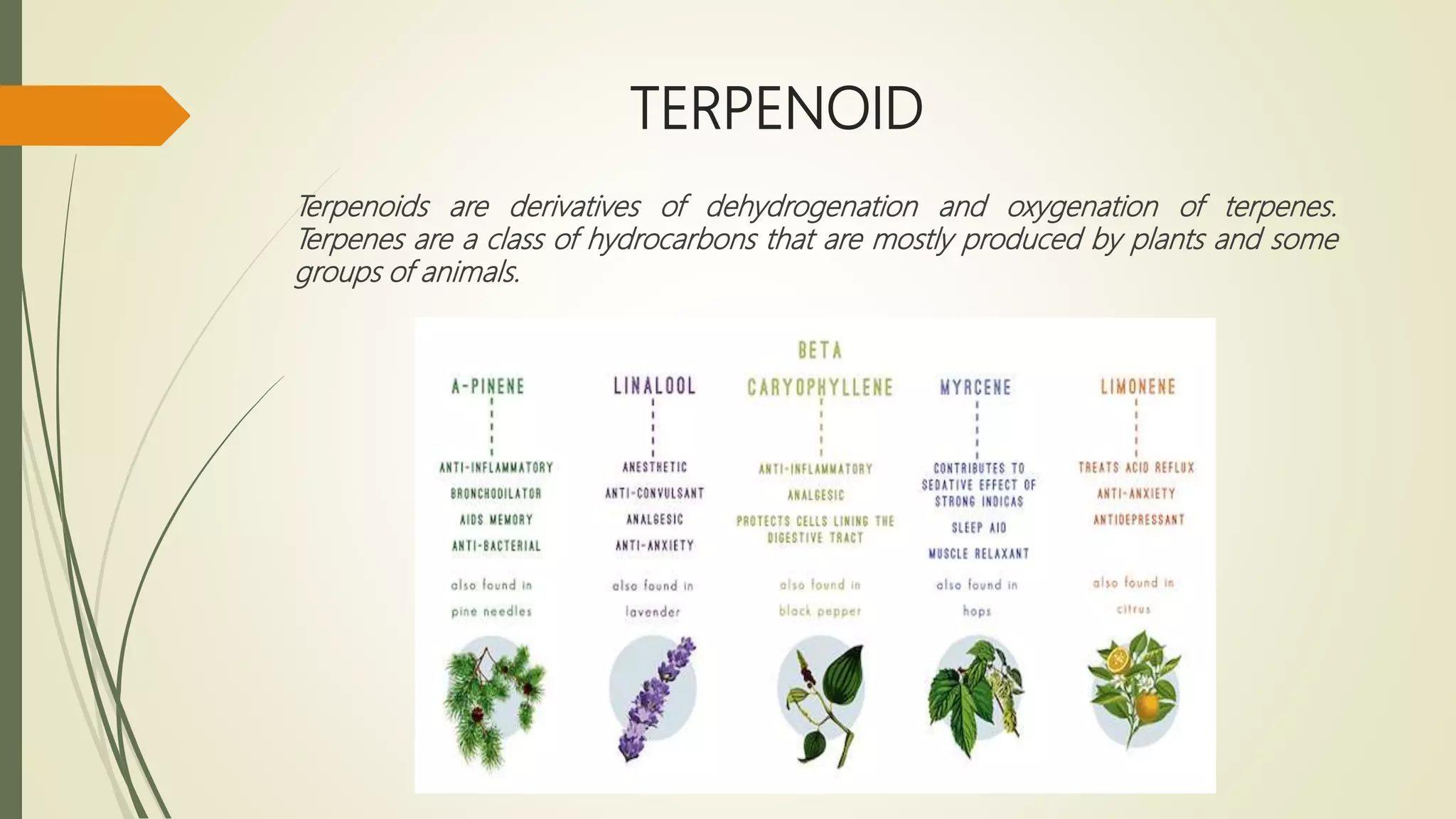
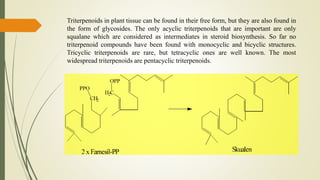
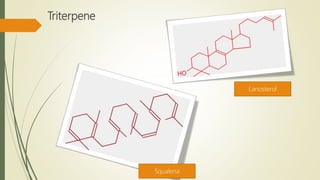
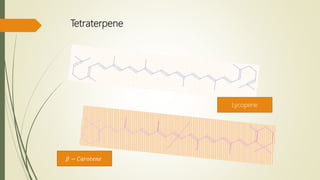
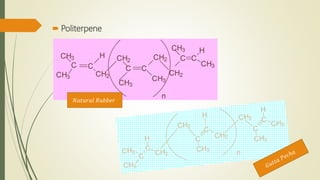
Terpenoids are derived from terpenes through dehydrogenation and oxygenation reactions. They are mostly produced by plants and some animal groups. Triterpenoids can be found freely or as glycosides in plant tissue, with pentacyclic triterpenoids being the most widespread. Tetraterpenes never form large ring structures and include carotenoids like lycopene and beta-carotene. Politerpenes are important polymers like natural rubber, which is thought to function as a carrier in plant biosynthesis.