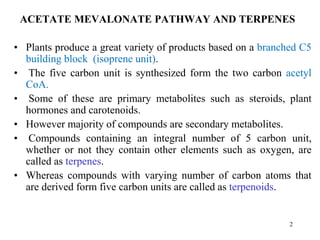
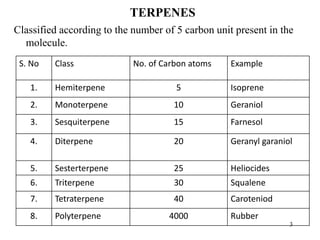
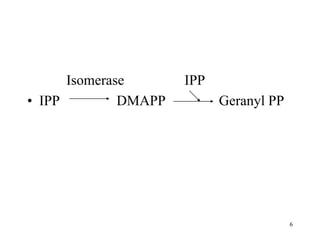
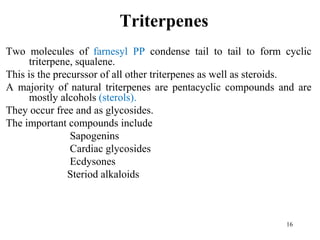
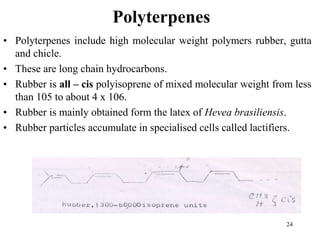
Terpenes are a class of compounds produced by plants derived from five-carbon isoprene units. They are classified based on the number of isoprene units present, from hemiterpenes with one unit to polyterpenes with many units. Terpenes include monoterpenes, sesquiterpenes, diterpenes, triterpenes, and tetraterpenes. They are synthesized via the mevalonate pathway and perform various important functions for plants such as constituents of essential oils, hormones, pigments, and defense compounds. Brassinosteroids are a newly identified class of plant growth regulating steroids.