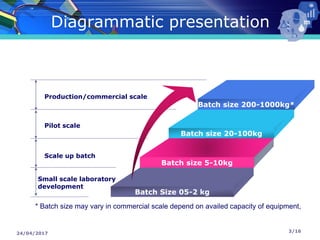
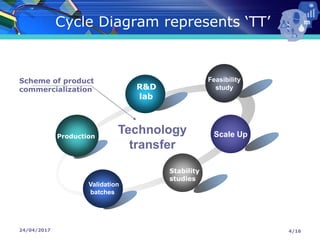
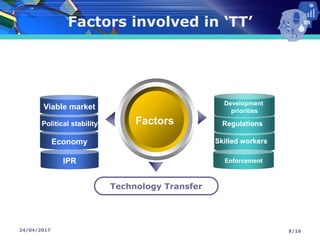
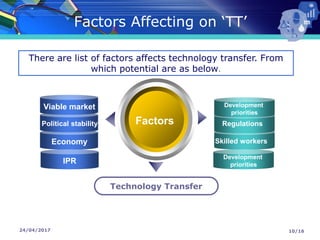
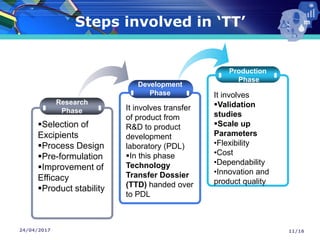
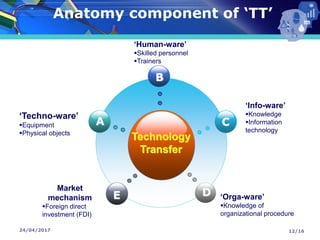
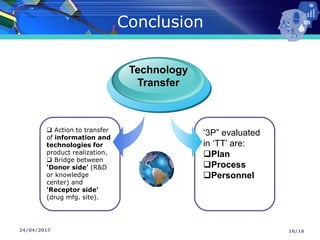
The document presents a seminar on technology transfer (TT) in the pharmaceutical industry, highlighting its significance in moving from drug discovery to commercialization. It outlines the definitions, importance, and various components of TT, including the phases involved and documentation required for successful implementation. Key factors such as government collaboration, market viability, and policies that govern TT practices are also discussed to emphasize its critical role in maintaining drug quality and reducing development costs.