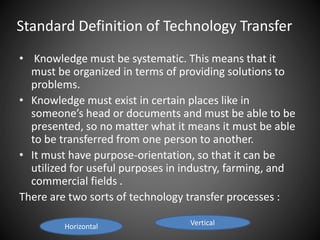
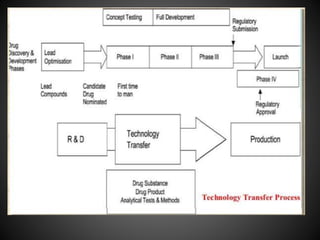
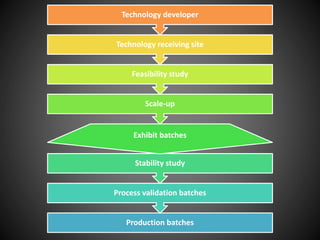
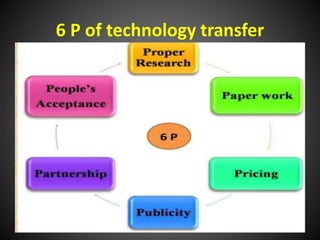
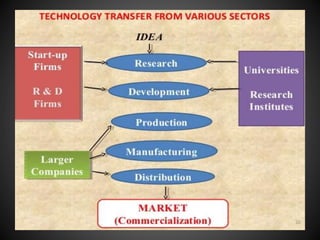
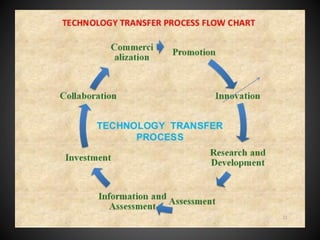
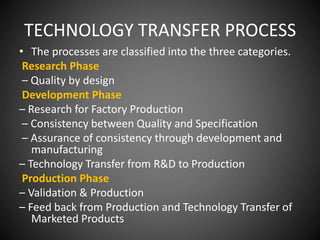
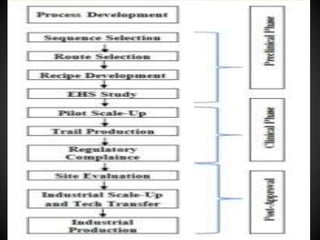
Technology transfer refers to the transfer of knowledge, processes, or technologies between different areas, such as research and development (R&D) to production. It is an important part of the drug development process, allowing processes to be scaled up from small laboratory batches to larger commercial production batches. There are various methods of technology transfer, including licensing, collaboration between government, academic, and private sectors, and transferring technology between private companies. Technology transfer helps move innovations beyond the lab and allows the benefits of research to reach society through commercialization. It is a key step in realizing the potential of new technologies and ensuring efficient development and manufacturing of quality products.