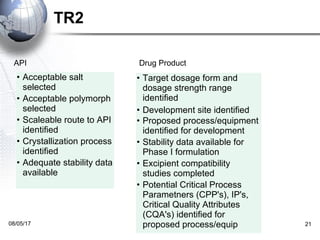
The document discusses the technology transfer process in the pharmaceutical industry, emphasizing the importance of governance systems and structured approaches to efficiently transfer knowledge from R&D to manufacturing. Key elements include technical reviews, product strategy assessments, and various tools and templates that facilitate the transfer while ensuring compliance and quality. The expected benefits of a structured process include improved efficiency, reduced cycle time, and enhanced predictability in costs and resources.