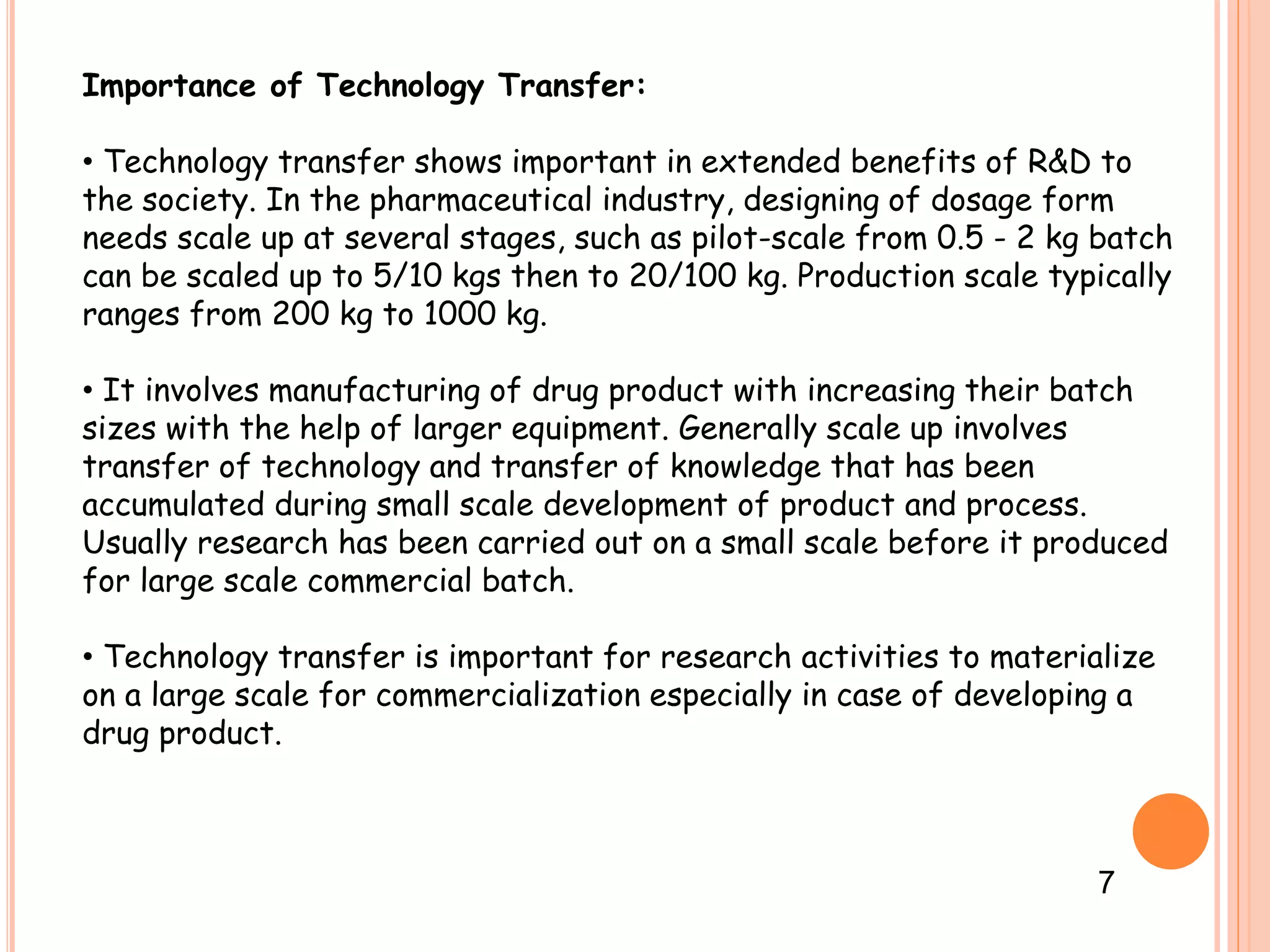
1. The document discusses technology transfer in the pharmaceutical industry, which refers to transferring details about formulations and analytical methods from research and development to production. This includes transferring a drug product from laboratory scale to production scale.
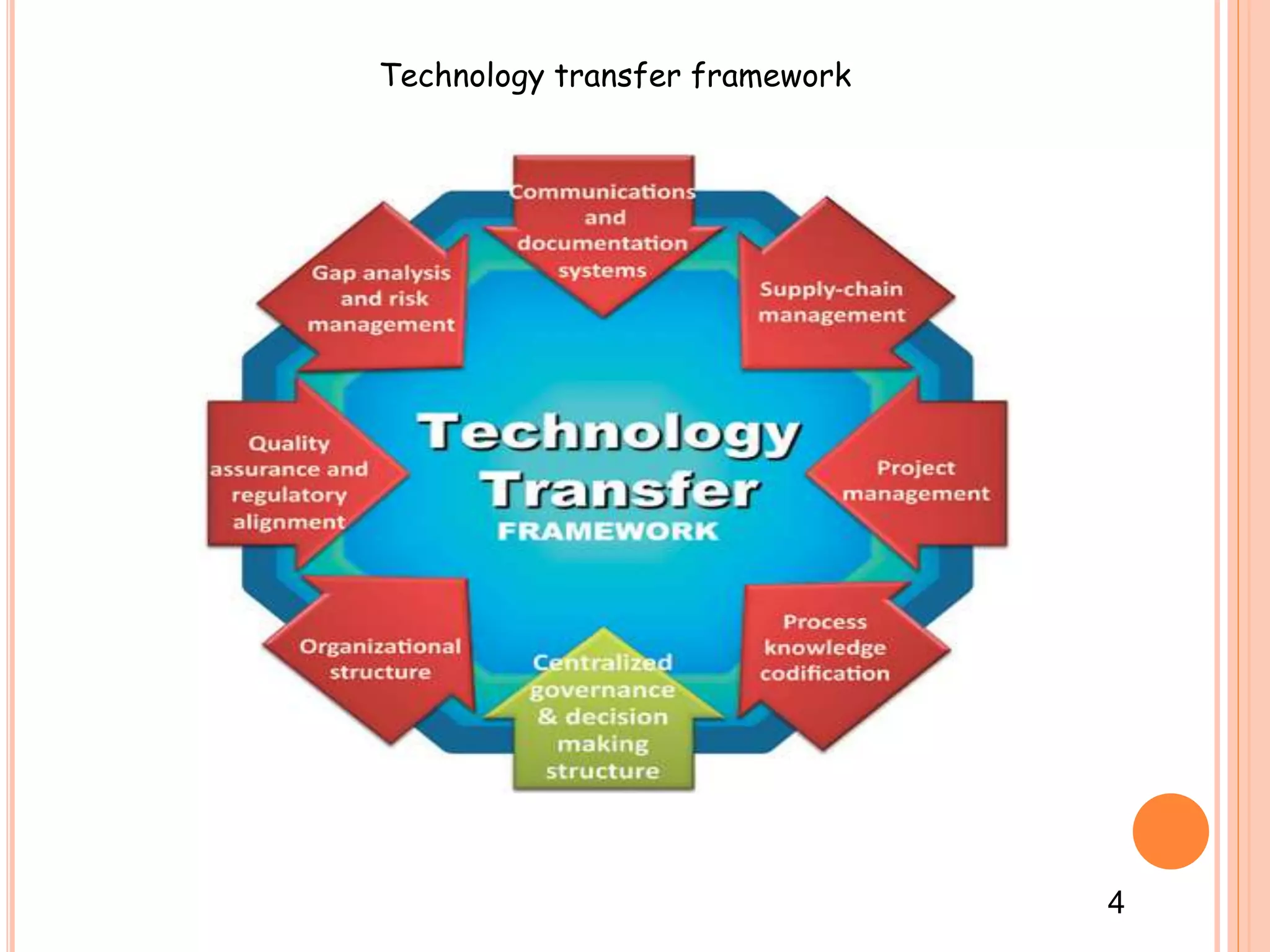
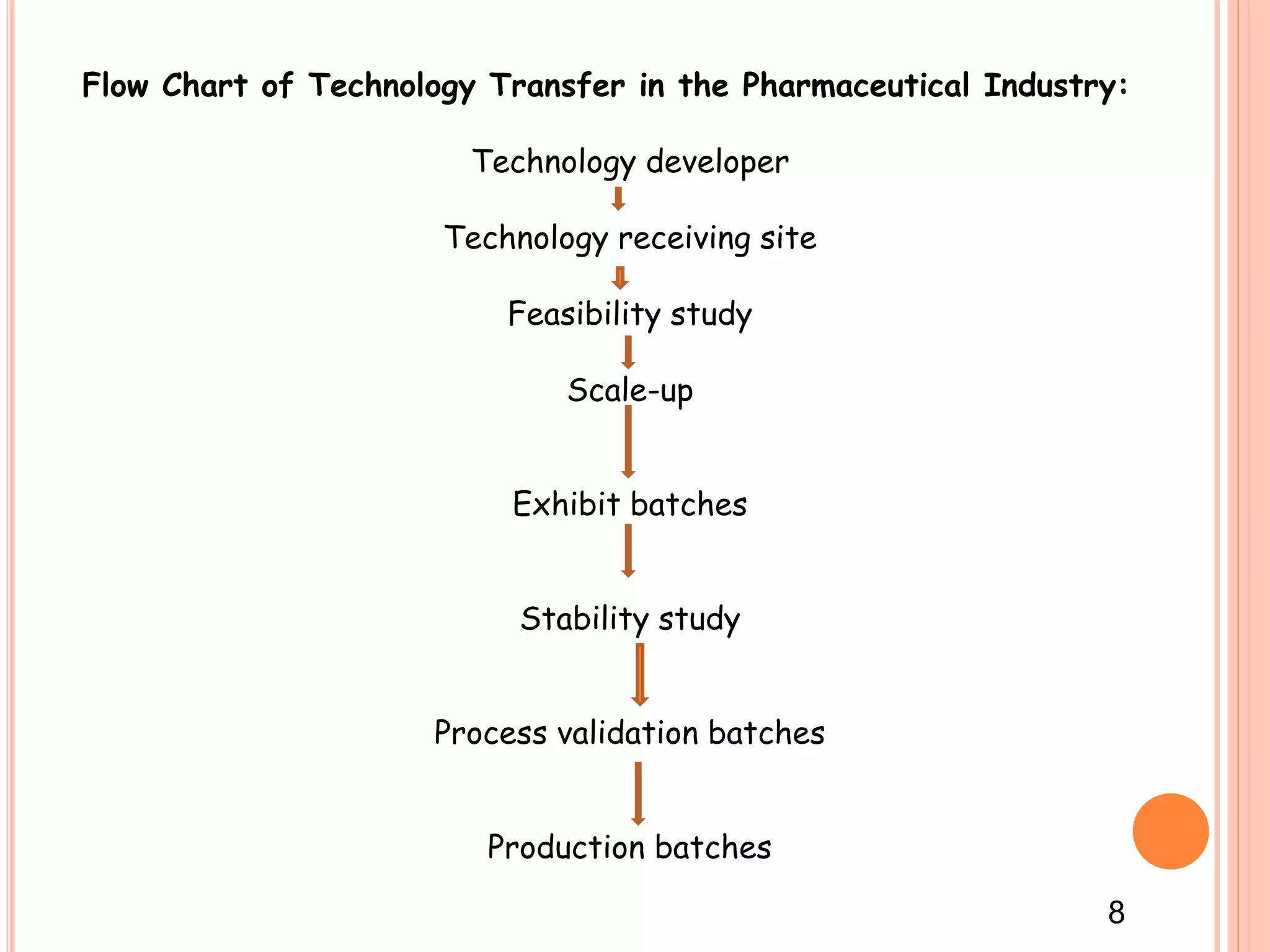
2. It outlines the goals, objectives, methods, and importance of technology transfer. The key methods are licensing and technology transfer involves a framework including a feasibility study, scale-up, validation, and production batches.
3. Successful technology transfer requires establishing a technology transfer team representing different areas like quality assurance, production, and engineering to review documentation, manufacturing processes, and equipment needs.