Embed presentation

![pH & pOH determined by the concentration of [H + ] in solution pH = - log [H + ] Conversely, [H + ] may be determined if pH of a solution is known. [H + ] = 10 -pH pH](https://image.slidesharecdn.com/tang03-phpoh2-111102143549-phpapp01/85/Tang-03-ph-poh-2-2-320.jpg)
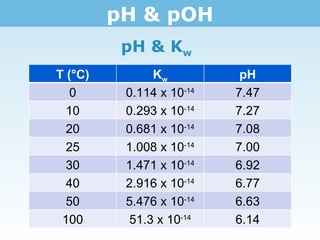
![pH & pOH pH of water From the K w of water @ 25°C (1.0 x 10 -14 ), we know that [H+] = 1.0 x 10 -7 . Why is the pH of water 7? Q: Is the pH of water always 7? Why or why not? Because the log of the above concentration is 7 No. pH is relative to temperature.](https://image.slidesharecdn.com/tang03-phpoh2-111102143549-phpapp01/85/Tang-03-ph-poh-2-3-320.jpg)
![pH & pOH pOH Similar to pH, pOH is determined by the concentration of [OH - ] in solution. pOH = - log [OH - ] [OH - ] = 10 -pOH If pH @ 25°C is known, how can pOH be determined? pH + pOH = 14](https://image.slidesharecdn.com/tang03-phpoh2-111102143549-phpapp01/85/Tang-03-ph-poh-2-5-320.jpg)
- pH is determined by the negative log of the hydrogen ion (H+) concentration in a solution, while pOH is determined by the negative log of the hydroxide ion (OH-) concentration. - The pH of water is 7 because at 25°C, the H+ concentration is 1.0 x 10-7 and the log of that value is 7. However, pH varies with temperature as the self-ionization constant of water (Kw) changes with temperature. - pOH can be calculated from pH using the relationship that pH + pOH must equal 14 for any aqueous solution.

![pH & pOH determined by the concentration of [H + ] in solution pH = - log [H + ] Conversely, [H + ] may be determined if pH of a solution is known. [H + ] = 10 -pH pH](https://image.slidesharecdn.com/tang03-phpoh2-111102143549-phpapp01/85/Tang-03-ph-poh-2-2-320.jpg)
![pH & pOH pH of water From the K w of water @ 25°C (1.0 x 10 -14 ), we know that [H+] = 1.0 x 10 -7 . Why is the pH of water 7? Q: Is the pH of water always 7? Why or why not? Because the log of the above concentration is 7 No. pH is relative to temperature.](https://image.slidesharecdn.com/tang03-phpoh2-111102143549-phpapp01/85/Tang-03-ph-poh-2-3-320.jpg)

![pH & pOH pOH Similar to pH, pOH is determined by the concentration of [OH - ] in solution. pOH = - log [OH - ] [OH - ] = 10 -pOH If pH @ 25°C is known, how can pOH be determined? pH + pOH = 14](https://image.slidesharecdn.com/tang03-phpoh2-111102143549-phpapp01/85/Tang-03-ph-poh-2-5-320.jpg)