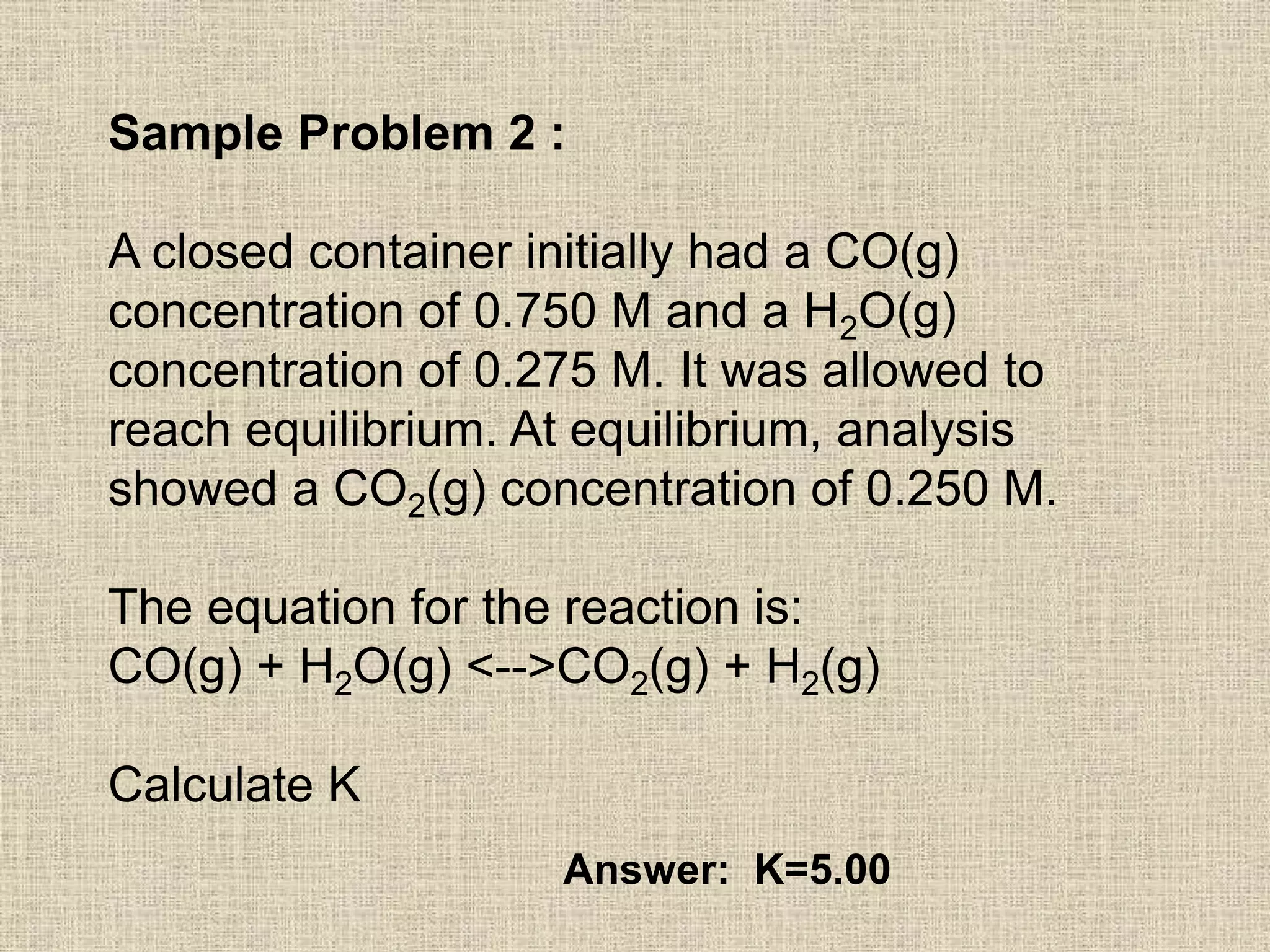
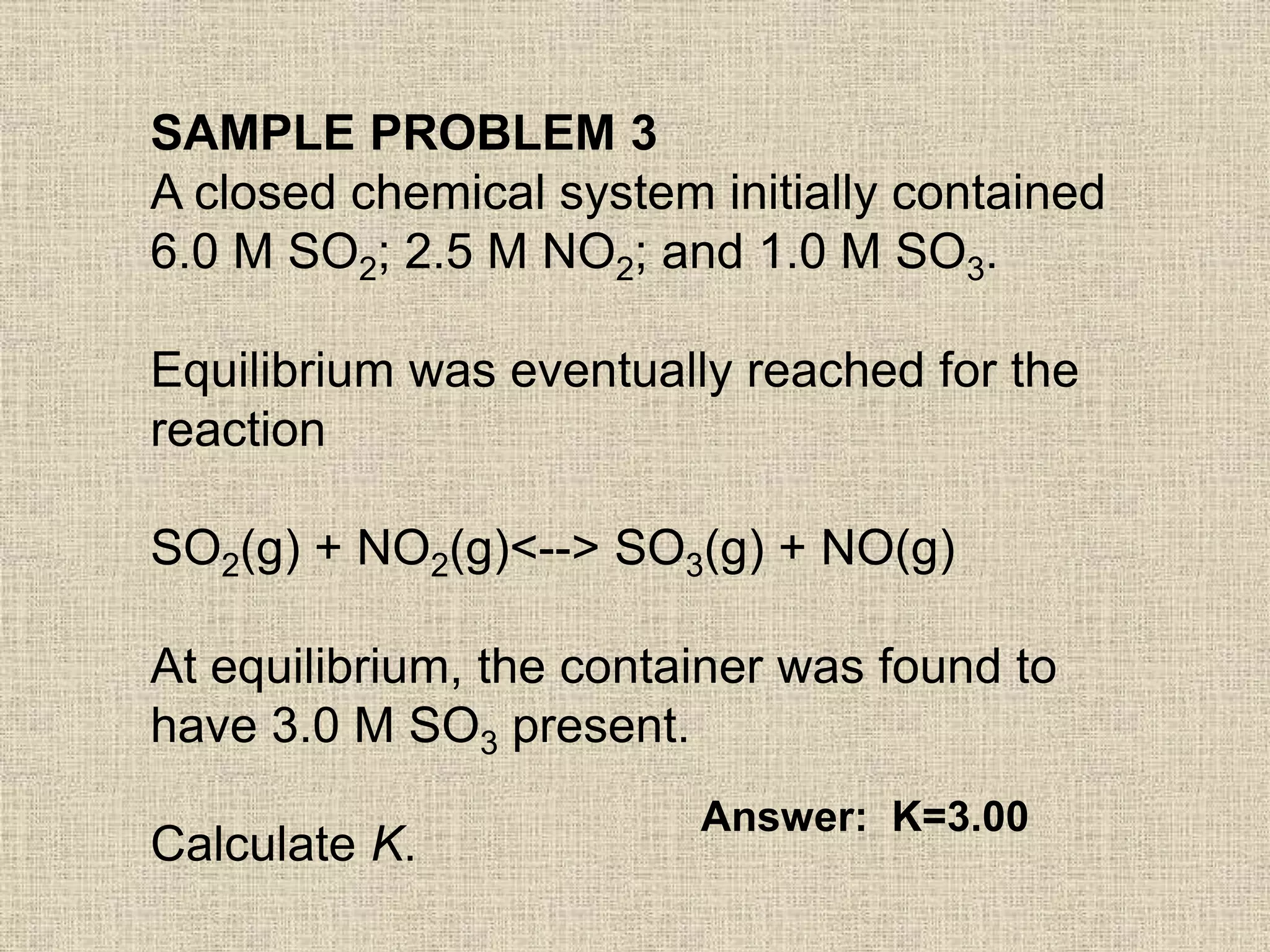
The document discusses equilibrium calculations using the equilibrium constant expression Kc. It provides examples of calculating concentrations at equilibrium using stoichiometry and initial concentrations. The examples then use the calculated equilibrium concentrations to determine the value of the equilibrium constant K for different reactions. The last example shows using the equilibrium expression to calculate the concentration of a product at equilibrium given initial concentrations and the value of K.



![Should Look like:
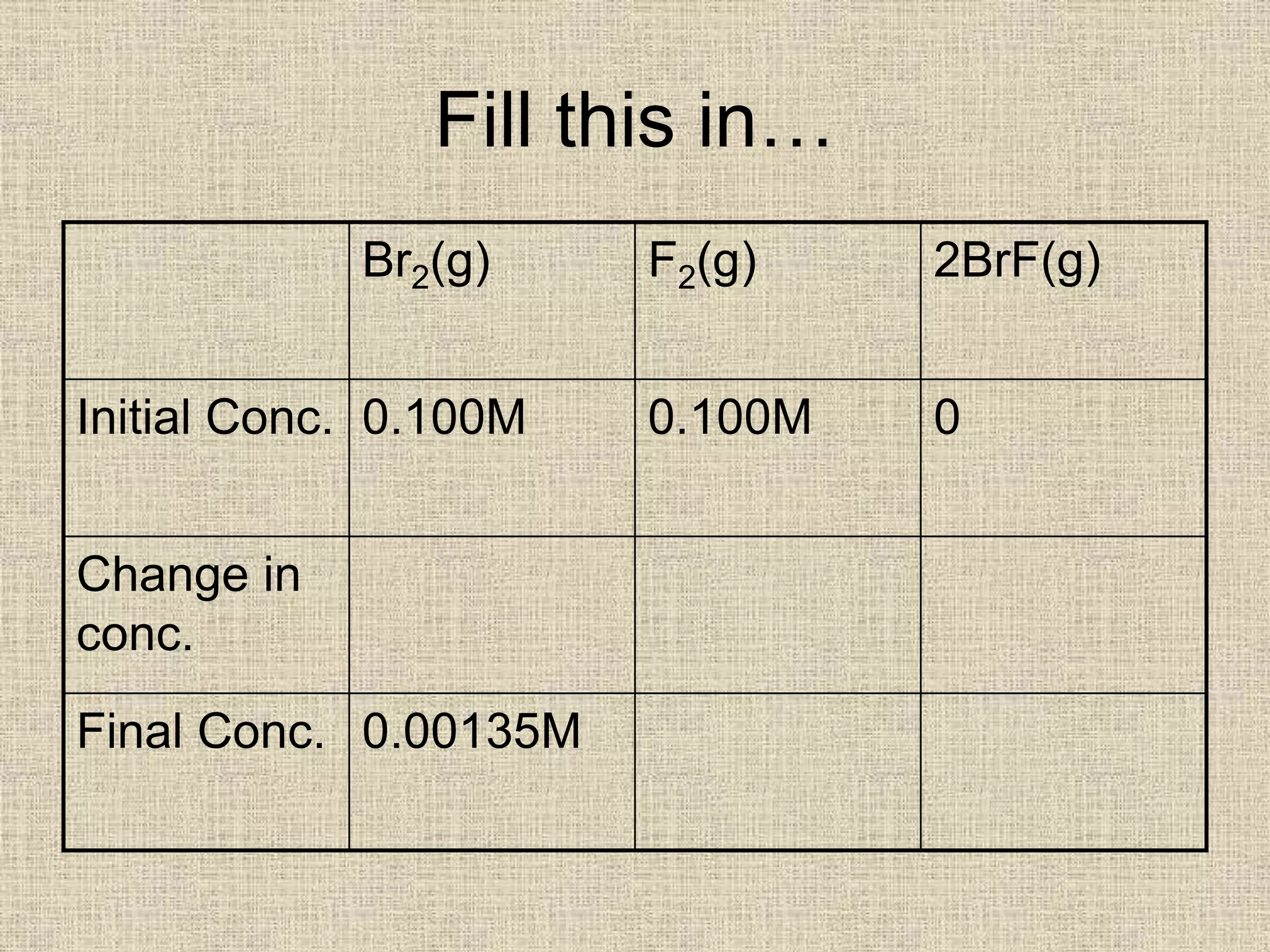
Br2(g) F2(g) 2BrF(g)
Initial Conc. 0.100M 0.100M 0
Change in
conc.
-0.09865M -0.09865M +[2 x
0.09865M]
=0.1973M
Final Conc. 0.00135M 0.00135M 0.1973M](https://image.slidesharecdn.com/equilibriumkccalculations-200323083123/75/Equilibrium-kc-calculations-5-2048.jpg)

![We’re not finished!
• Now we can solve for K because we found
the final concentrations (at equilibrium)
• K =[BrF]2 / [Br2][F2]
=[0.1973M]2/ [0.00135M][0.00135M]
=2.15 x 104](https://image.slidesharecdn.com/equilibriumkccalculations-200323083123/75/Equilibrium-kc-calculations-7-2048.jpg)

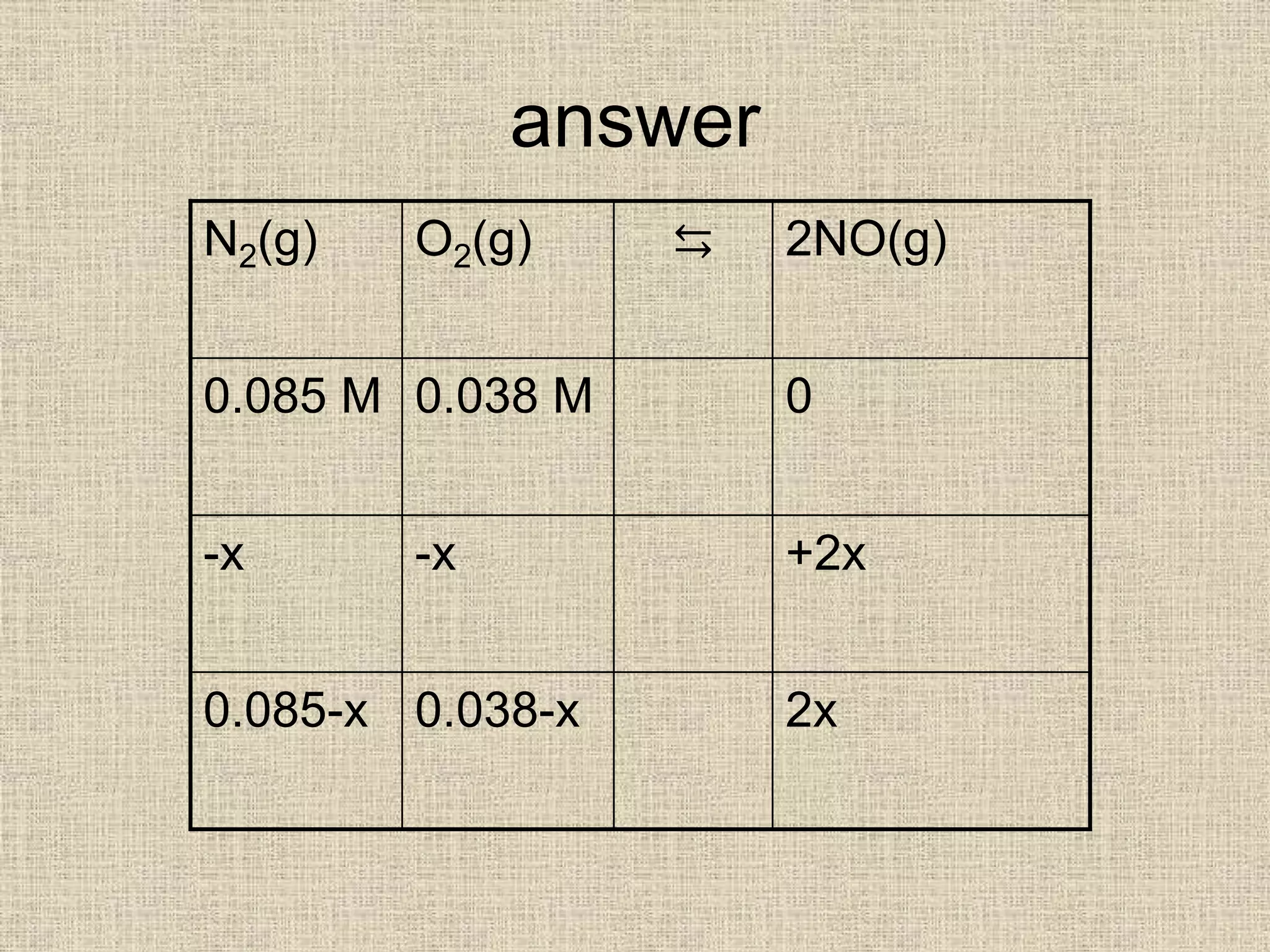

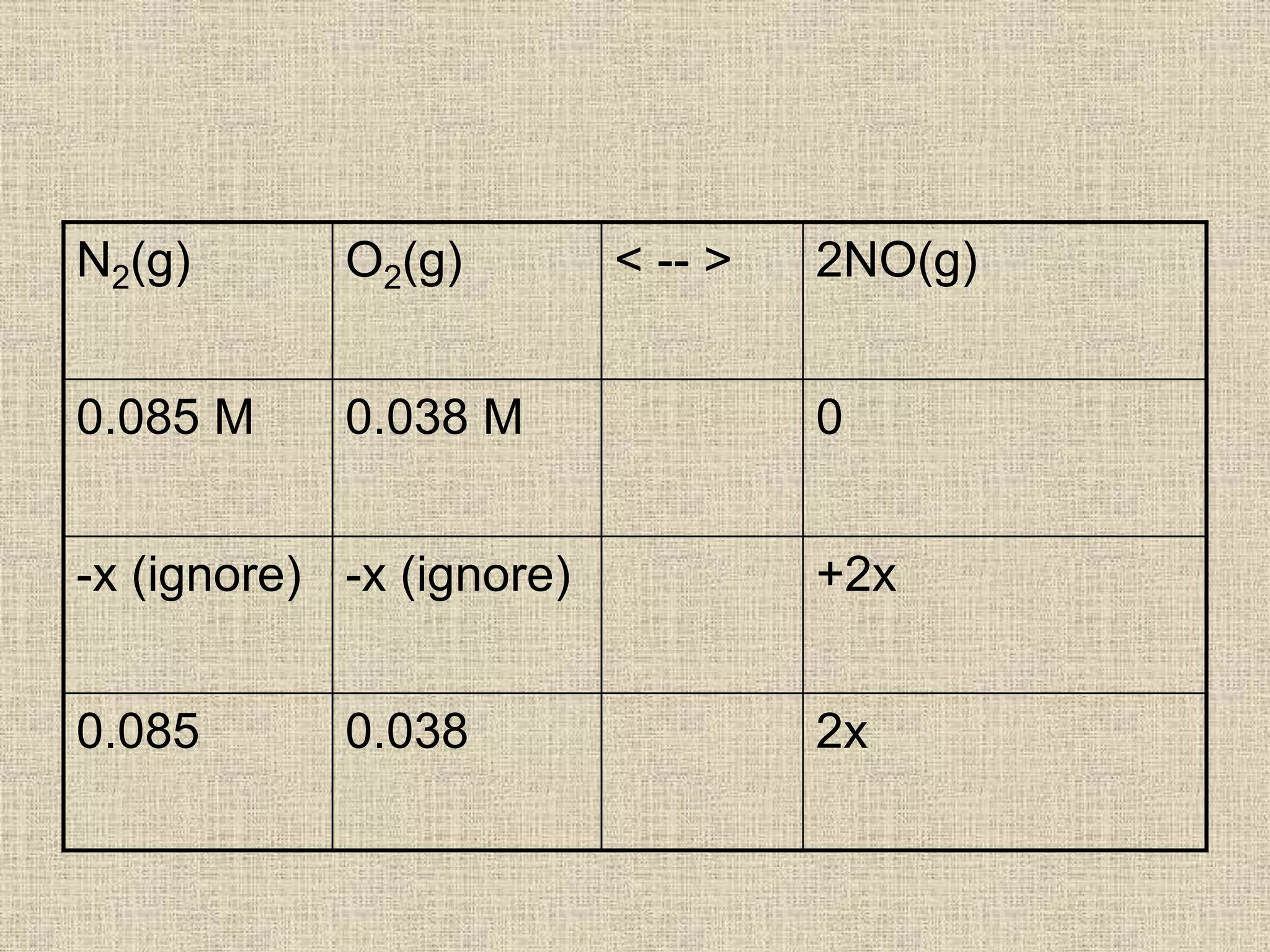
![• k = [2x]2 =4.2 x10-8
[0.085][0.038]
4x2 = (4.2x10-8) x [0.085] x [0.038]
4x2 = 1.357 x 10-10
x2 = 3.392 x 10-11
x = 5.82 x 10-6
[NO] = 2x = 2 x 5.82 x10-6 = 1.2 x10-5 M](https://image.slidesharecdn.com/equilibriumkccalculations-200323083123/75/Equilibrium-kc-calculations-14-2048.jpg)


