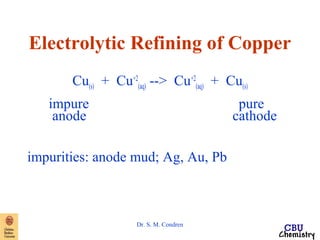
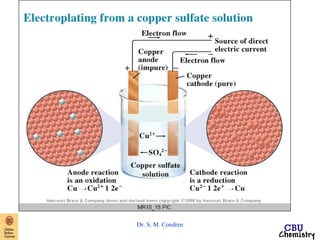
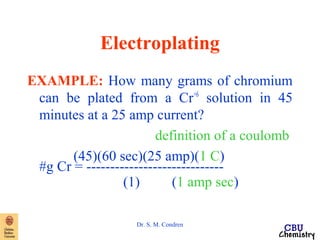
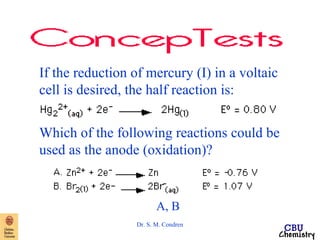
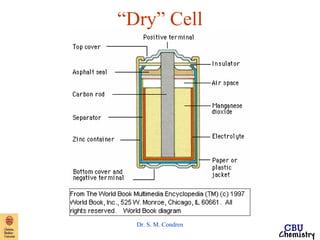
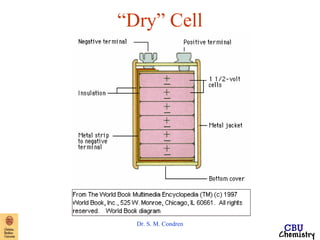
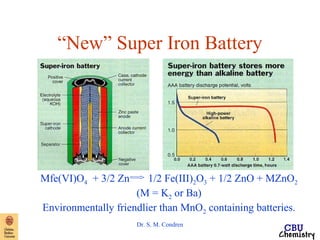
This document summarizes key concepts from an electrochemistry chapter, including:
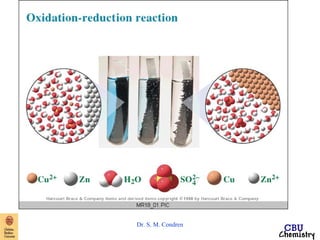
- Redox reactions involve the gain or loss of electrons. Voltaic cells harness redox reactions to produce electric currents.
- Standard reduction potentials indicate the tendency of half-reactions to gain or lose electrons under standard conditions.
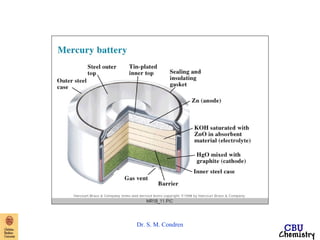
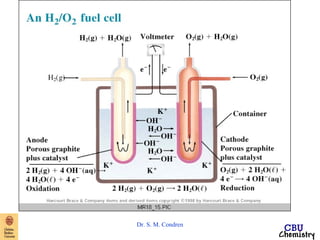
- Electrochemical cells use spontaneous redox reactions to generate voltage. Applications include batteries, electrolysis, corrosion prevention, and pH meters.





















![Automobile Oxygen Sensor
ZrO2 / CaO
Dr. S. M. Condren
Air,
constant [O 2 ]
porous Pt electrodes
migrating
O 2 - ions
exhaust gas,
unknown [O2 ]
measured potential difference](https://image.slidesharecdn.com/c115c1801electrochemistry-141015062841-conversion-gate01/85/Electrochemistry-33-320.jpg)


![Effect of Concentration on Cell
Voltage: The Nernst Equation
Ecell = Eo
cell - (RT/nF)ln Q
Ecell = Eo
cell - (0.0592/n)log Q
where Q => reaction quotient
Q = [products]/[reactants]
Dr. S. M. Condren](https://image.slidesharecdn.com/c115c1801electrochemistry-141015062841-conversion-gate01/85/Electrochemistry-36-320.jpg)
![EXAMPLE: What is the cell potential for the
Daniel's cell when the [Zn+2] = 10 [Cu+2] ?
Q = ([Zn+2]/[Cu+2] = (10 [Cu+2])/[Cu+2] = 10
Eo = (0.34 V)Cu couple + (-(-0.76 V)Zn couple
n = 2, 2 electron change Ecell = Eo
cell - (0.0257/n)ln Q
thus Ecell = (1.10 - (0.0257/2)ln 10) V
Ecell = (1.10 - (0.0257/2)2.303) V
Ecell = (1.10 - 0.0296) V = 1.07 V
Dr. S. M. Condren](https://image.slidesharecdn.com/c115c1801electrochemistry-141015062841-conversion-gate01/85/Electrochemistry-37-320.jpg)
![Nernst Equation
nF ln Q = – 2.3 RT
F log[H+]base side
[H+]acid side
H 2 in
1 atm
e– e–
Dr. S. M. Condren
+0.83 V
salt bridge
H 2 in
1 atm
voltmeter
Pt
electrode
Pt
NaOH electrode
1 M
anode (–)
HCl
1 M
cathode (+)
F log [h+]n-type side
[h+]p-type side
n p +
+
+
+
–
–
–
–
[H+]acid side ® [H+]base side
E = Eo – RT
[h+]p-type side ® [h+]n-type side
E (in volts) = – 2.3 RT](https://image.slidesharecdn.com/c115c1801electrochemistry-141015062841-conversion-gate01/85/Electrochemistry-38-320.jpg)