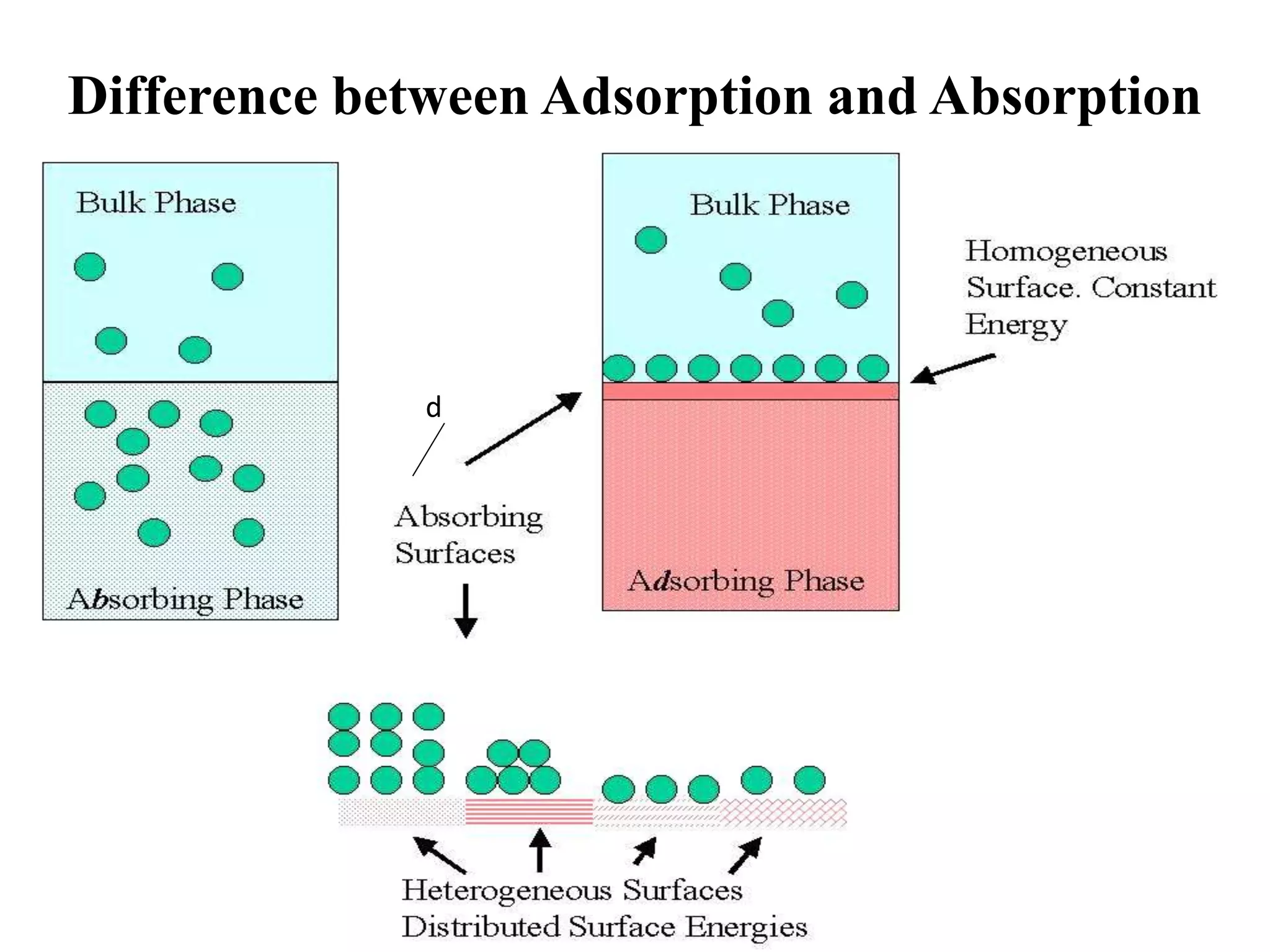
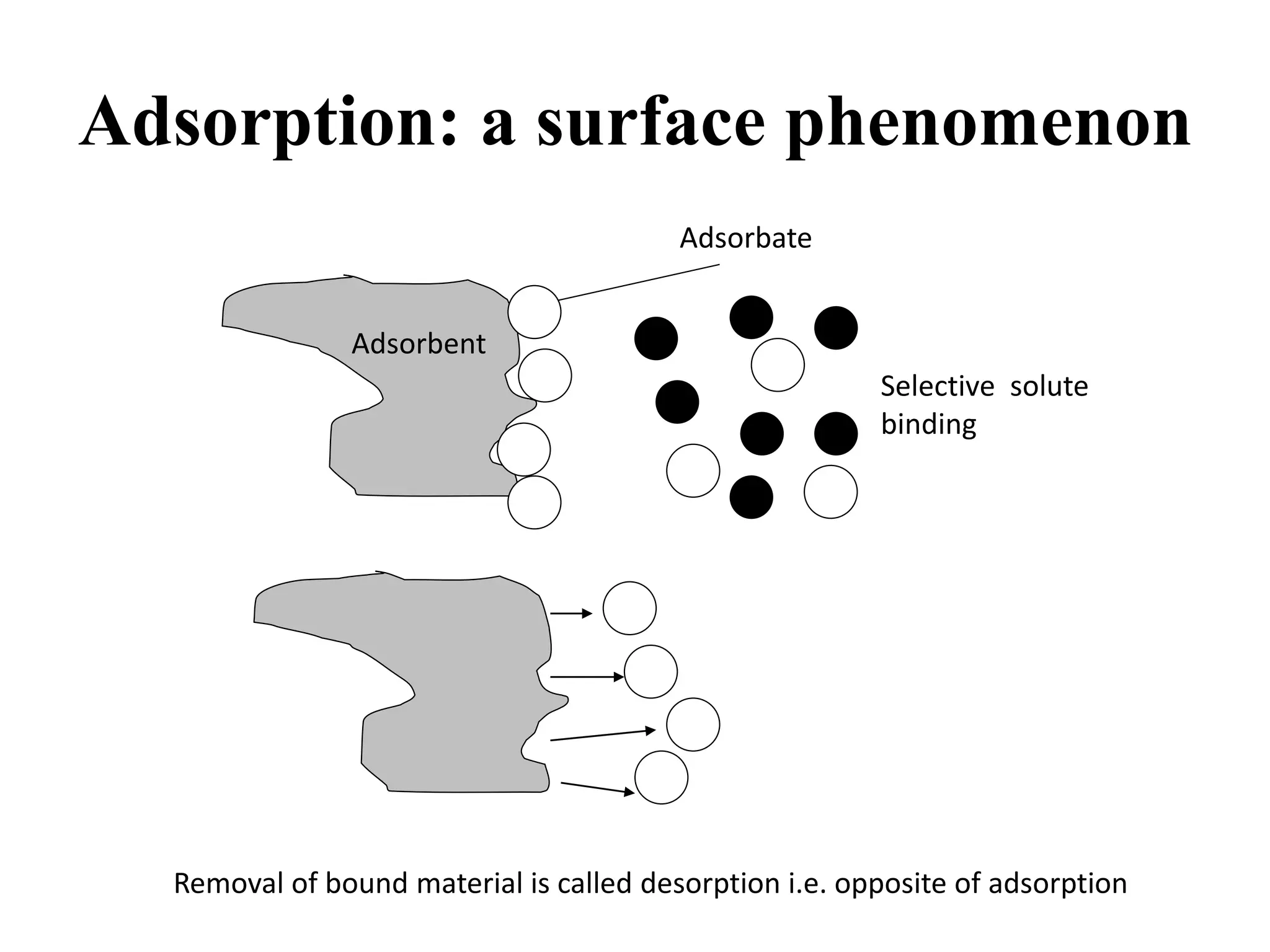
Adsorption is the process where a gas or liquid accumulates on the surface of a solid or liquid, forming a film. It occurs due to surface energy - atoms on the surface experience a bond deficiency. There are two types: physisorption, where adsorbate adheres via weak van der Waals forces; and chemisorption, where chemical bonds form. Factors influencing adsorption include surface area, gas nature, temperature, and pressure. Adsorption has applications in vacuum production, gas masks, catalysis, and water purification.