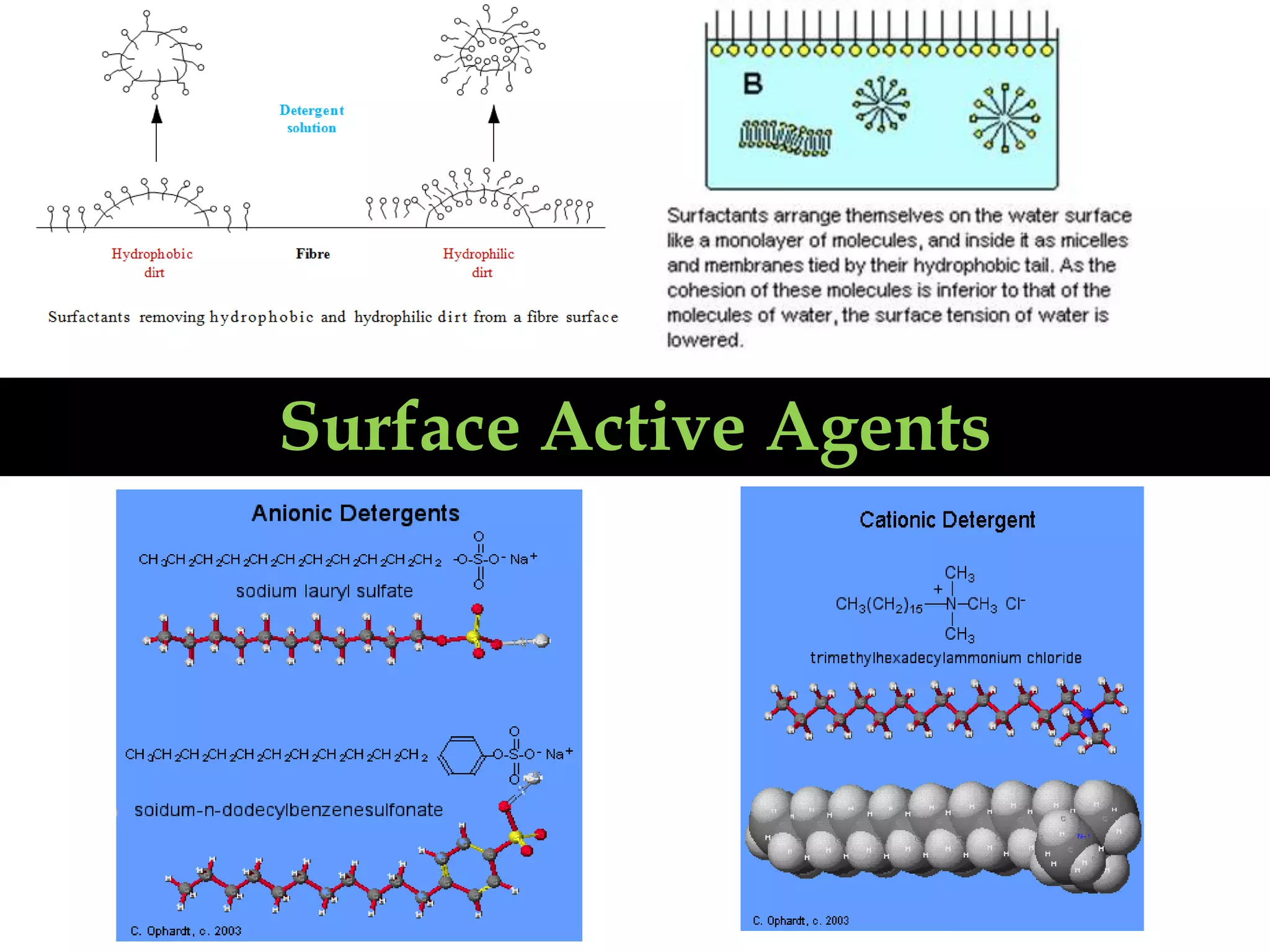
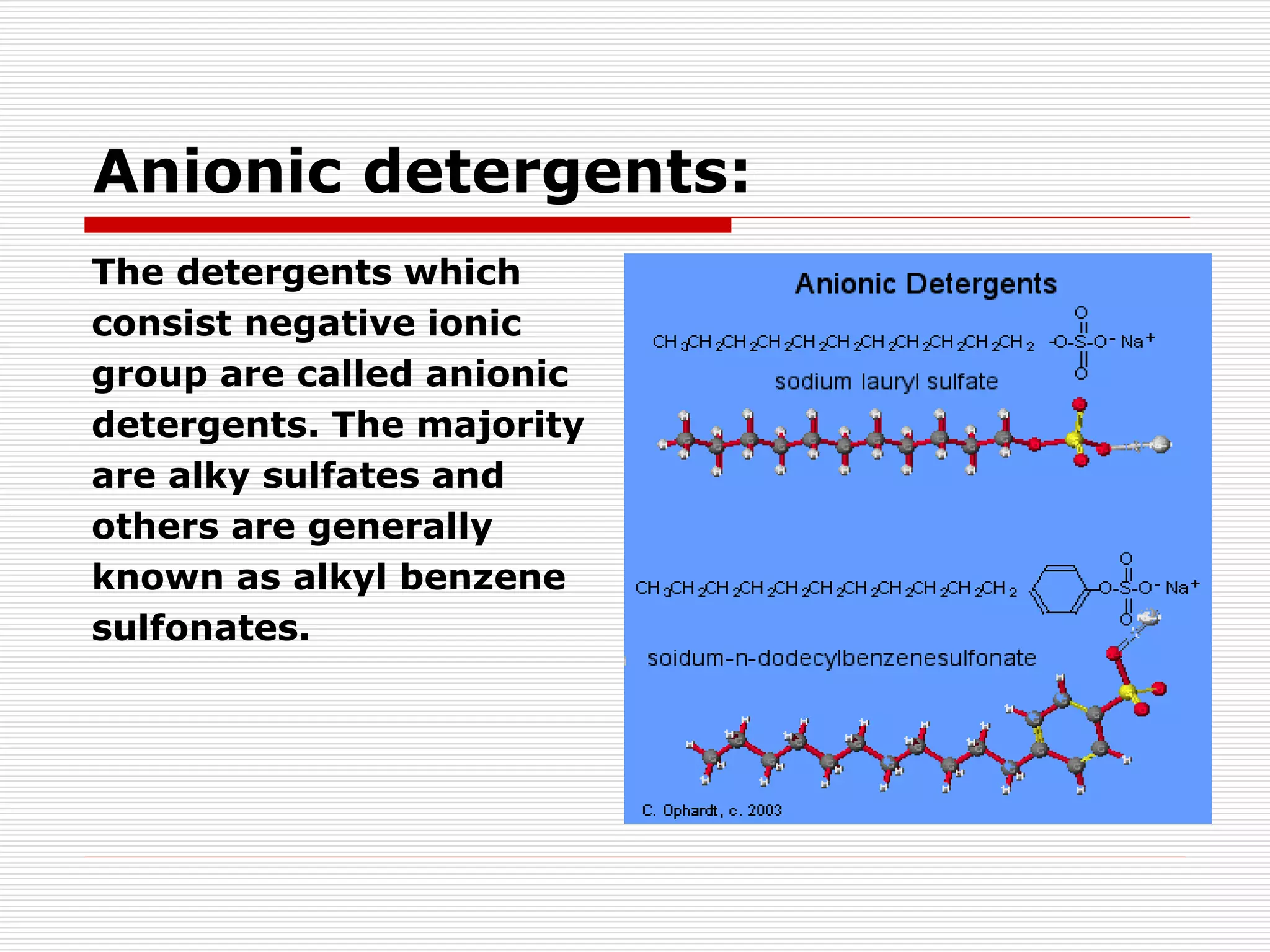
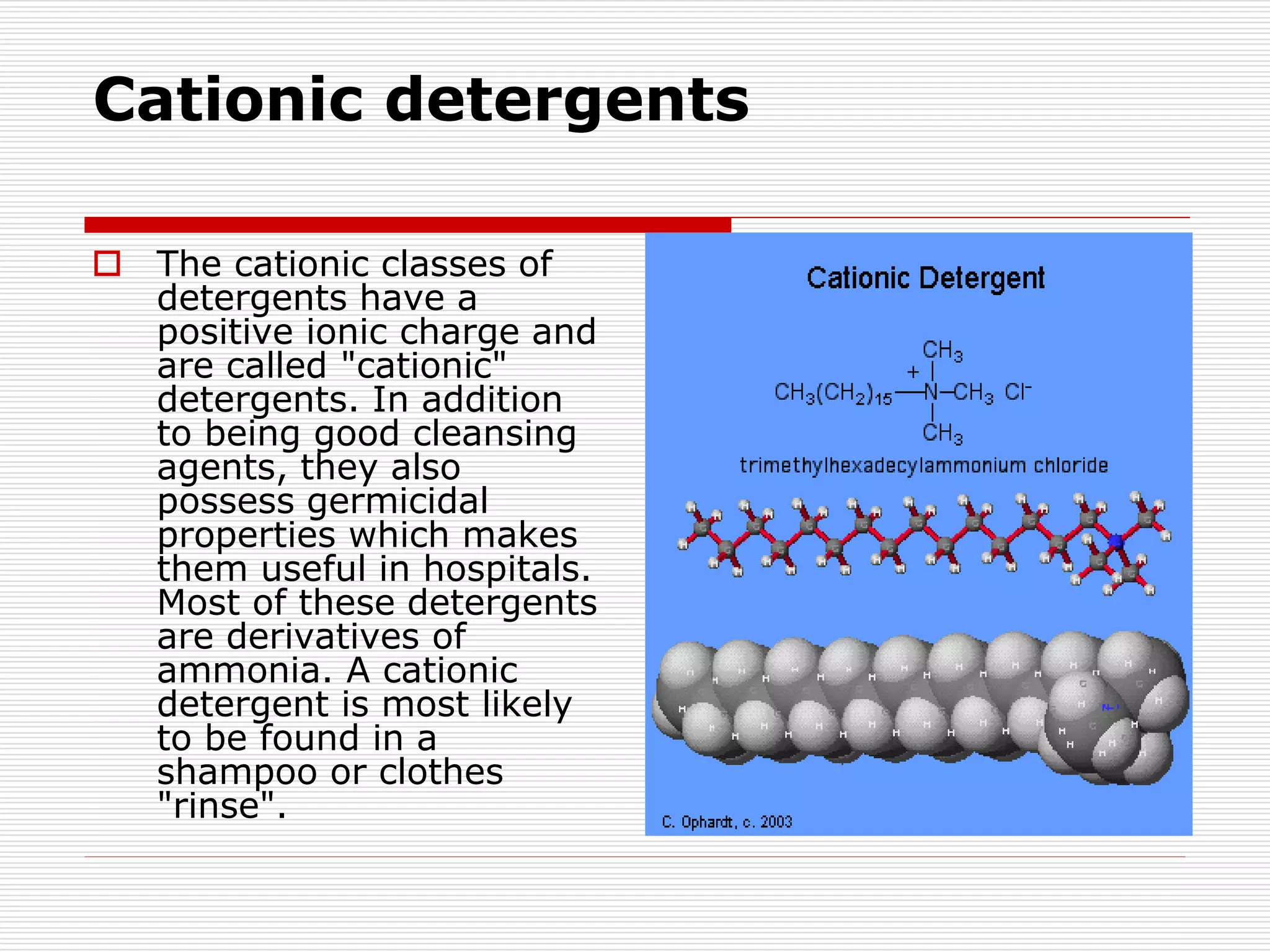
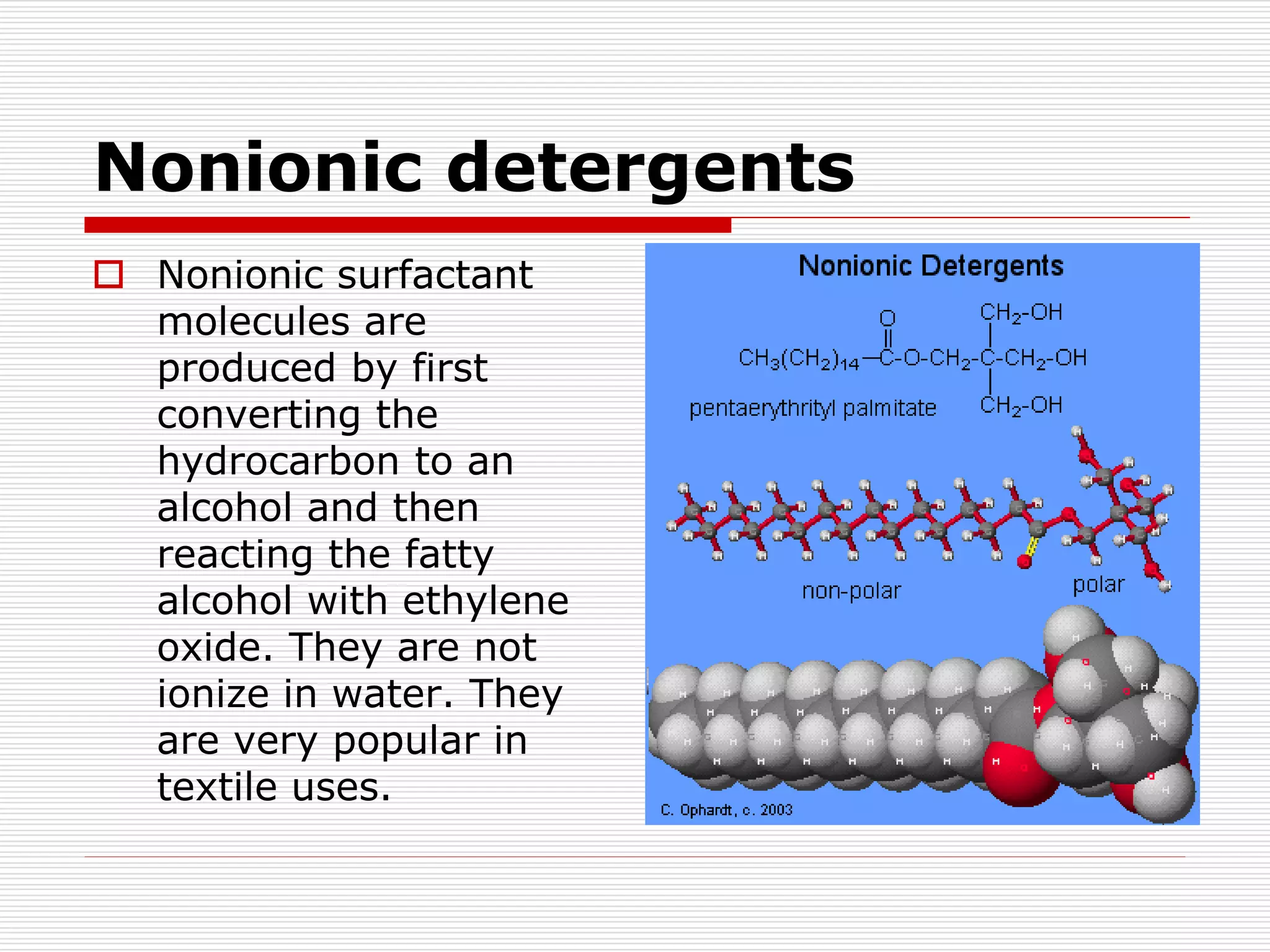
The document discusses surface active agents, also known as surfactants. Surfactants are amphiphilic organic compounds that contain both hydrophobic and hydrophilic groups. When added to a liquid, surfactants reduce surface tension and increase spreading and wetting properties. In textile dyeing, surfactants help dye penetrate fabric evenly. Surfactants have various applications including detergents, fabric softeners, emulsifiers, paints, adhesives, and more. Detergents are classified as ionic (anionic, cationic, amphoteric) or nonionic. Anionic detergents contain negative ions like alkyl sulfates or alkyl benzene sulfonates. Cationic deterg