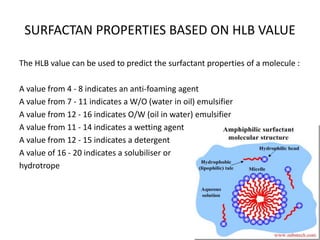
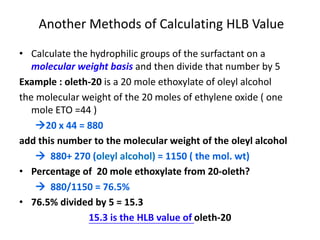
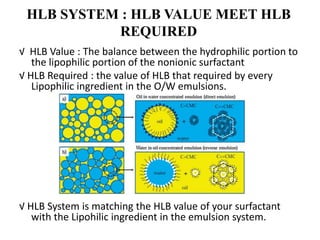
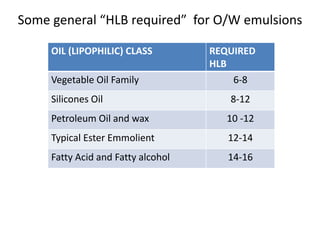
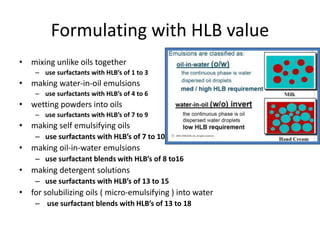
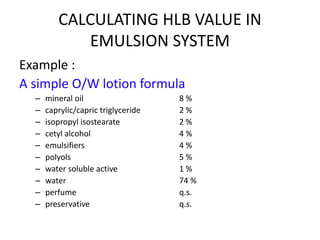
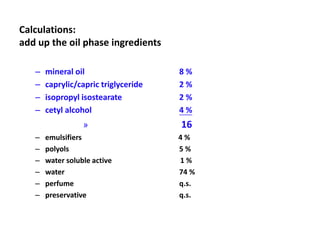
The document discusses the Hydrophile-Lipophile Balance (HLB) system, which quantifies the balance between the hydrophilic and lipophilic parts of nonionic surfactants to guide emulsification performance. It explains how to calculate HLB values and their corresponding applications in various emulsification processes and surfactant choices. Additionally, it provides examples and recommendations for formulating emulsions based on the calculated HLB values of ingredients.