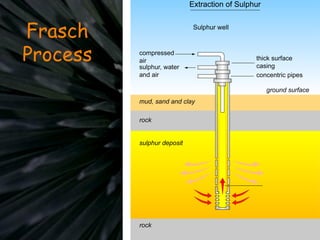
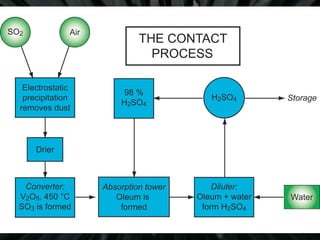
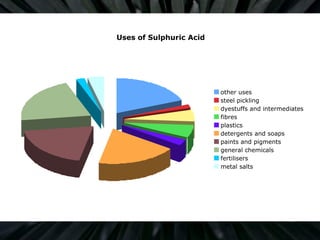
The document details the contact process for manufacturing sulfuric acid from sulfur(IV) oxide (SO2) through oxidation to sulfur(VI) oxide (SO3). It outlines various sources of SO2, reaction conditions for optimal conversion, and the role of vanadium(V) oxide as a catalyst. Additionally, it discusses the applications of sulfuric acid and its related compounds in several industries.