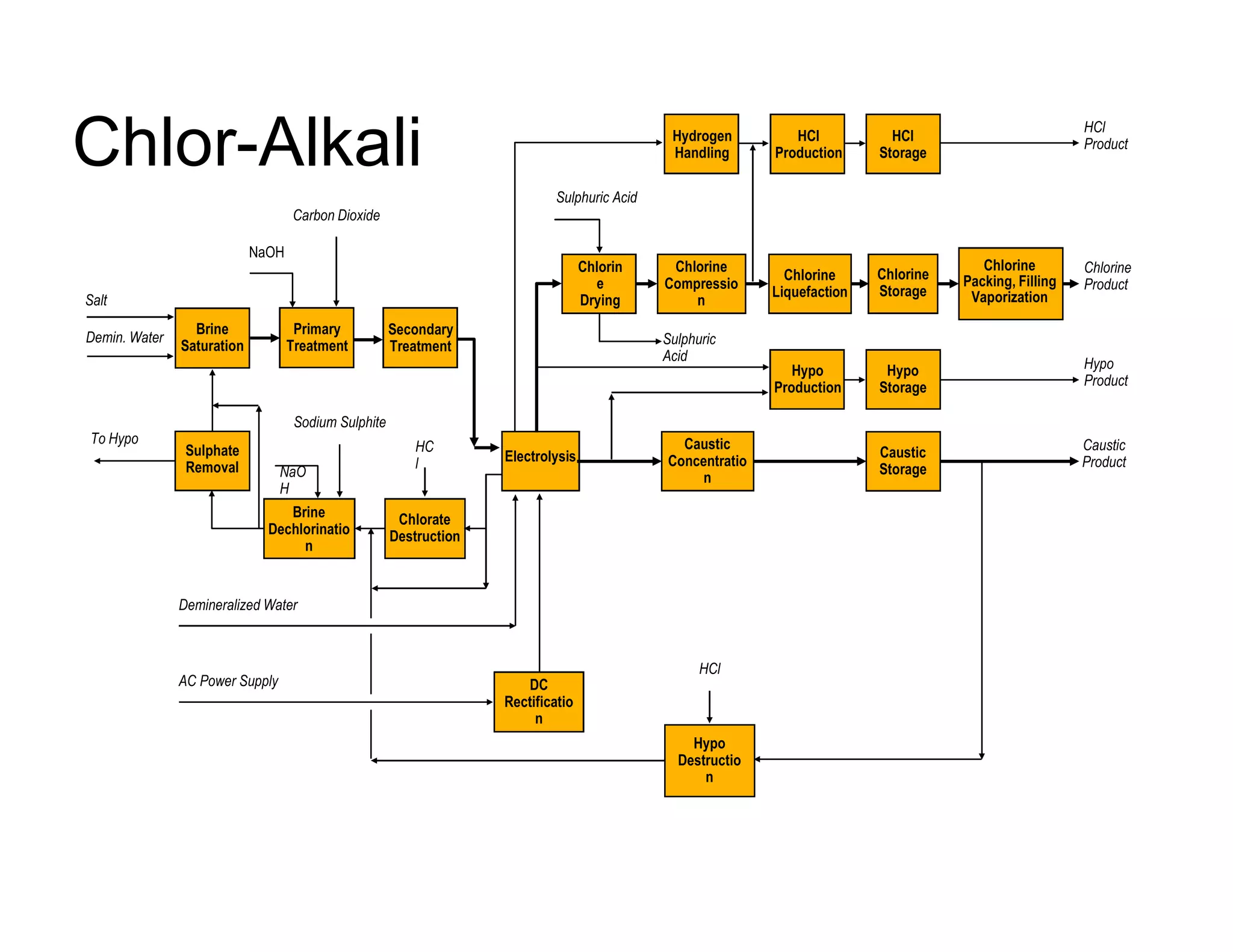
The document describes the chlor-alkali process for producing chlorine and sodium hydroxide through the electrolysis of sodium chloride brine. Key aspects include:
- Sodium chloride brine is purified through processes like precipitation to remove impurities before electrolysis.
- During electrolysis, chlorine gas is produced at the anode, sodium hydroxide at the cathode, and hydrogen as a byproduct. A membrane separates the anode and cathode compartments.
- Weak brine leaving the anode contains dissolved chlorine which is removed through processes like acidification before recycling. Sodium hydroxide product is cooled and may be concentrated.