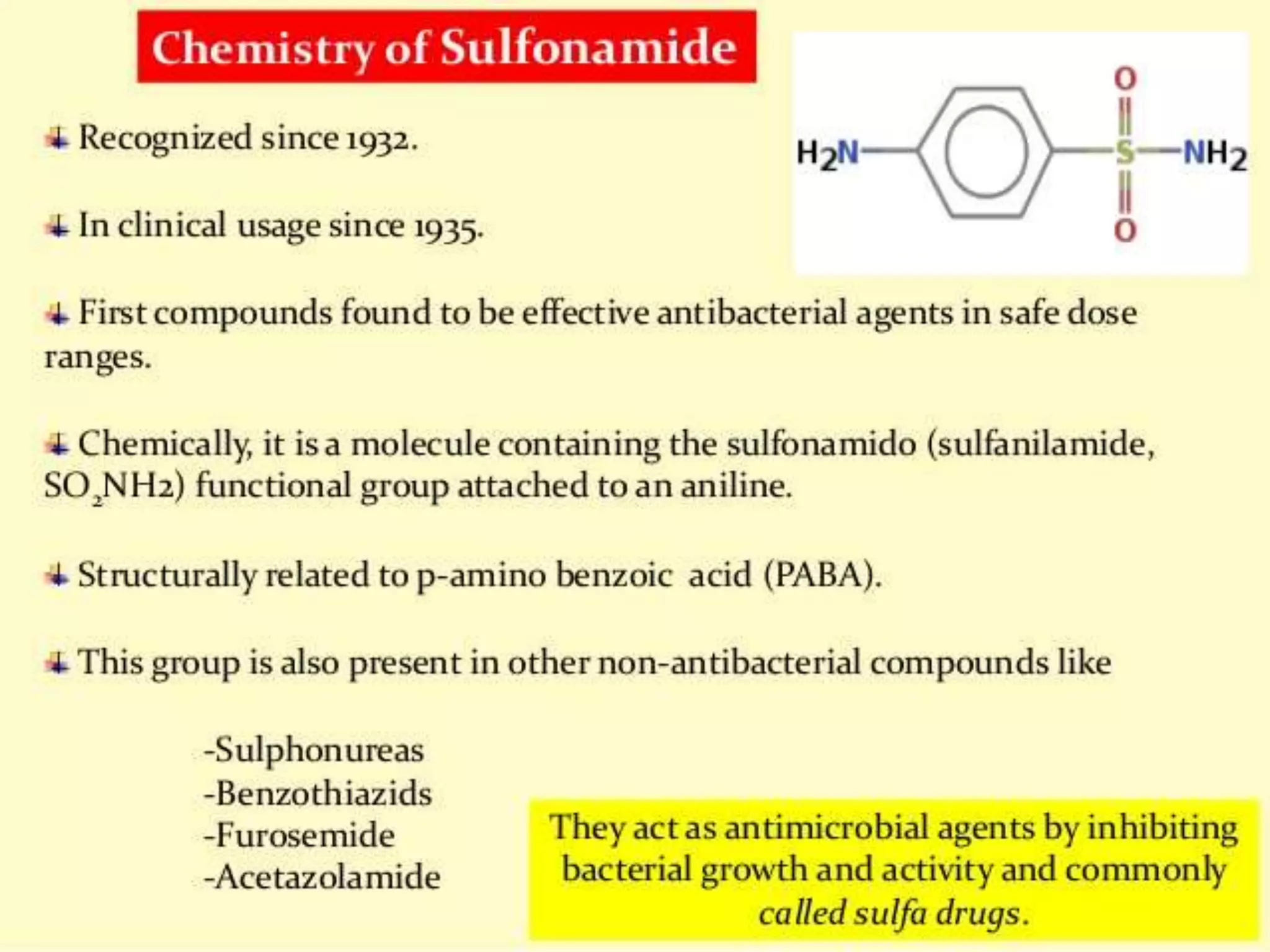
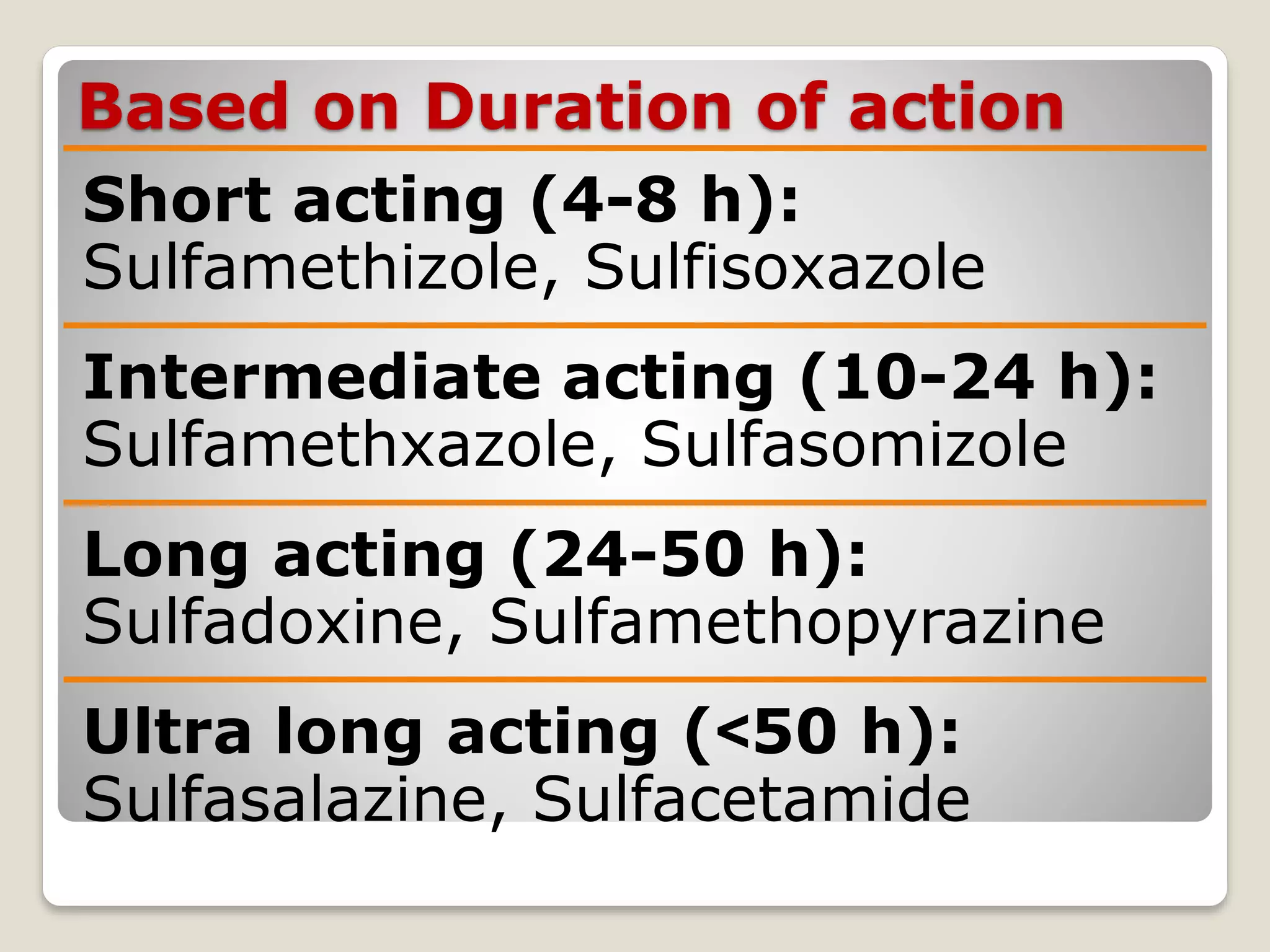
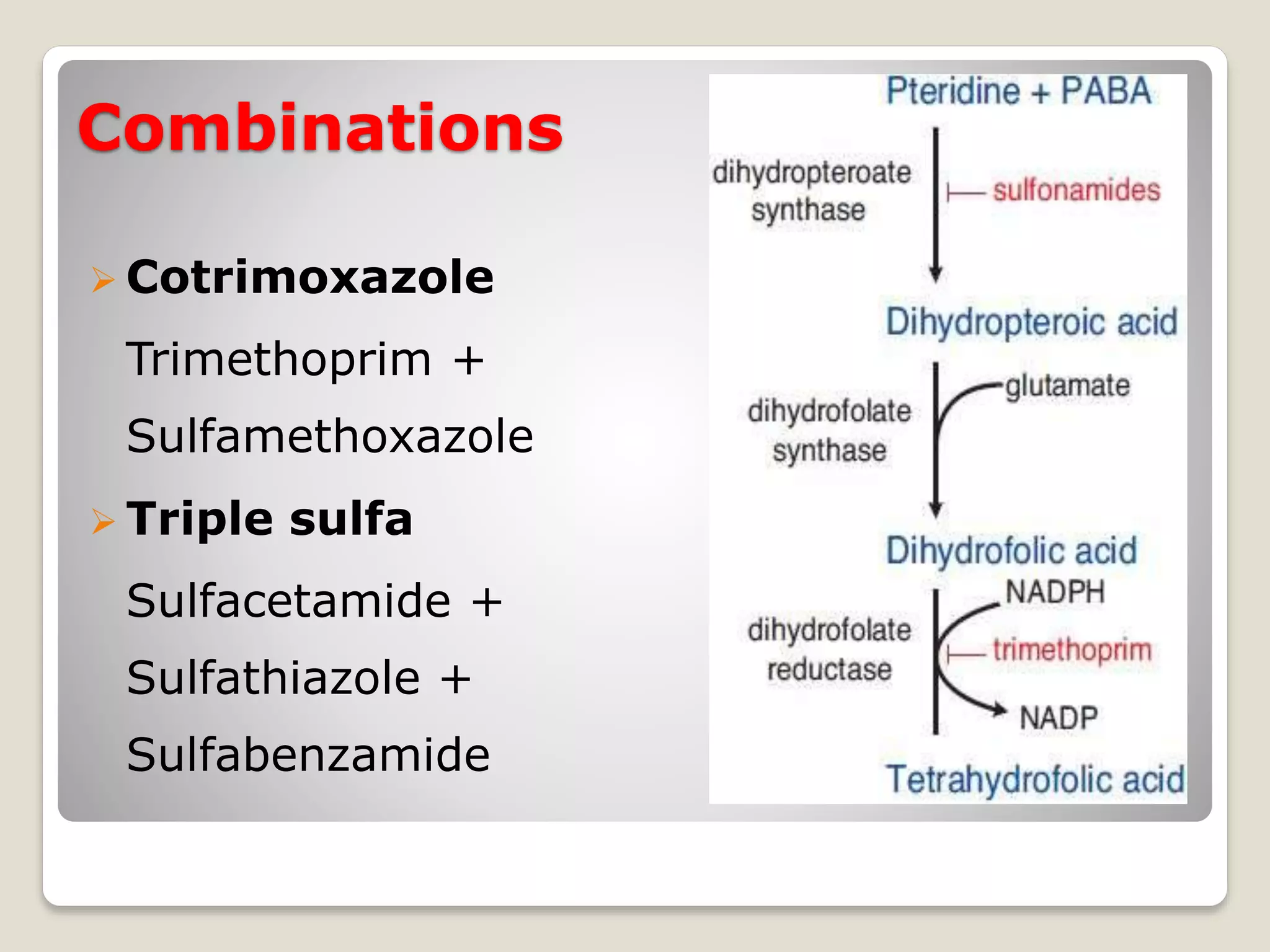
The document discusses sulfonamides, including their discovery by Gerhard Domagk, modes of classification based on duration of action, pharmacokinetics, chemistry, and mechanisms of action. It also covers their therapeutic uses such as for urinary tract infections, respiratory infections, and combinations including cotrimoxazole and triple sulfa.