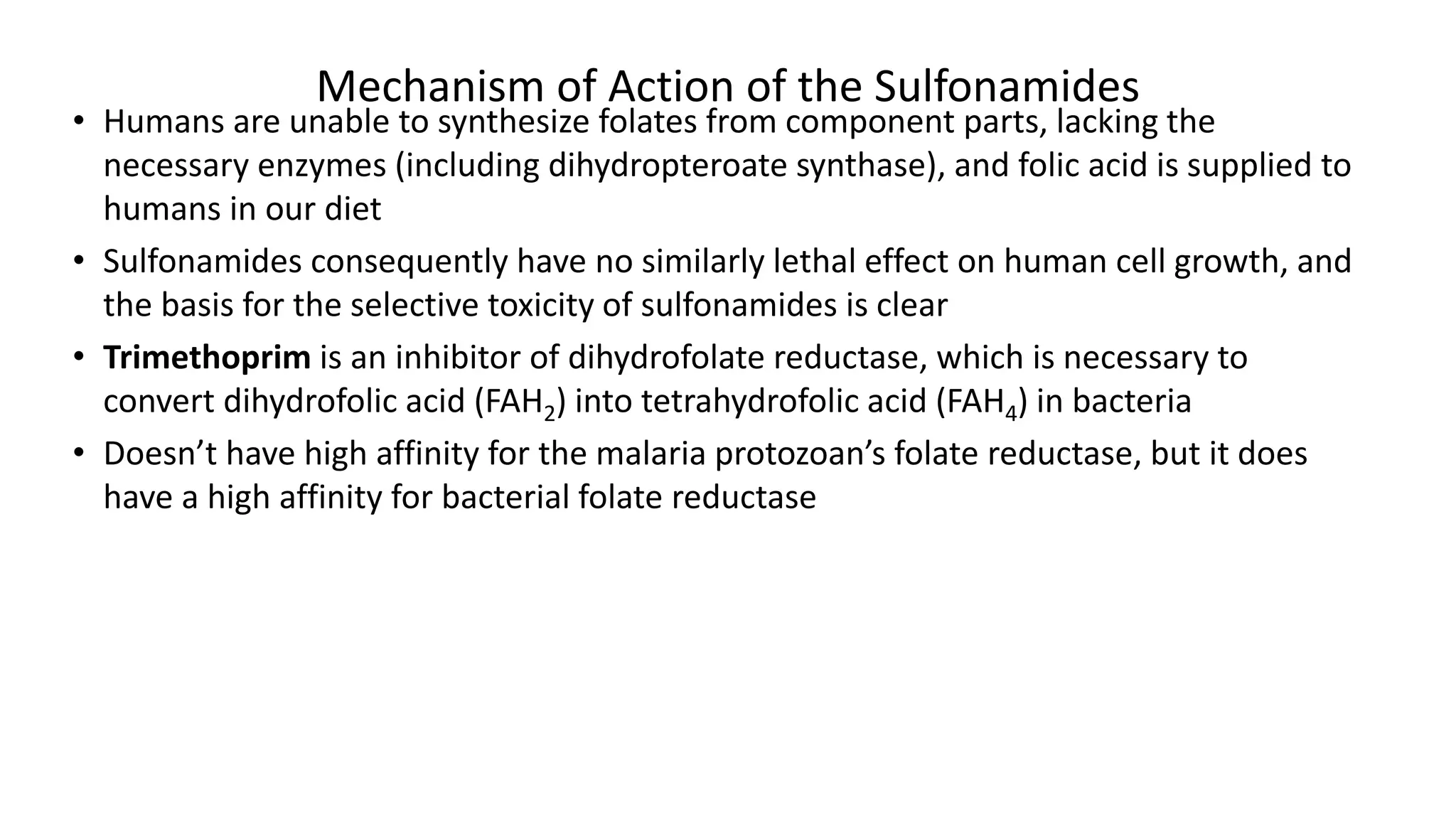
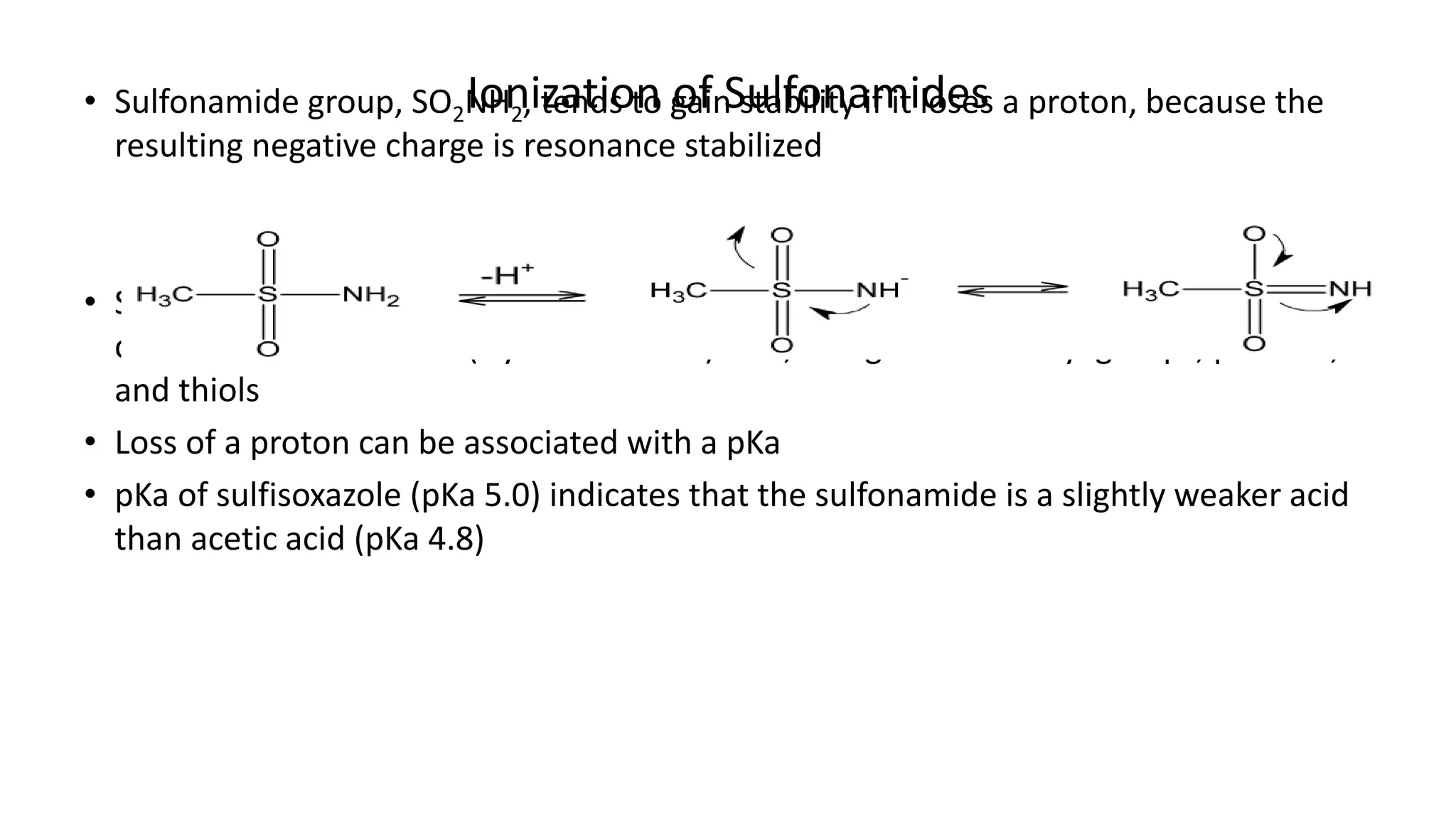
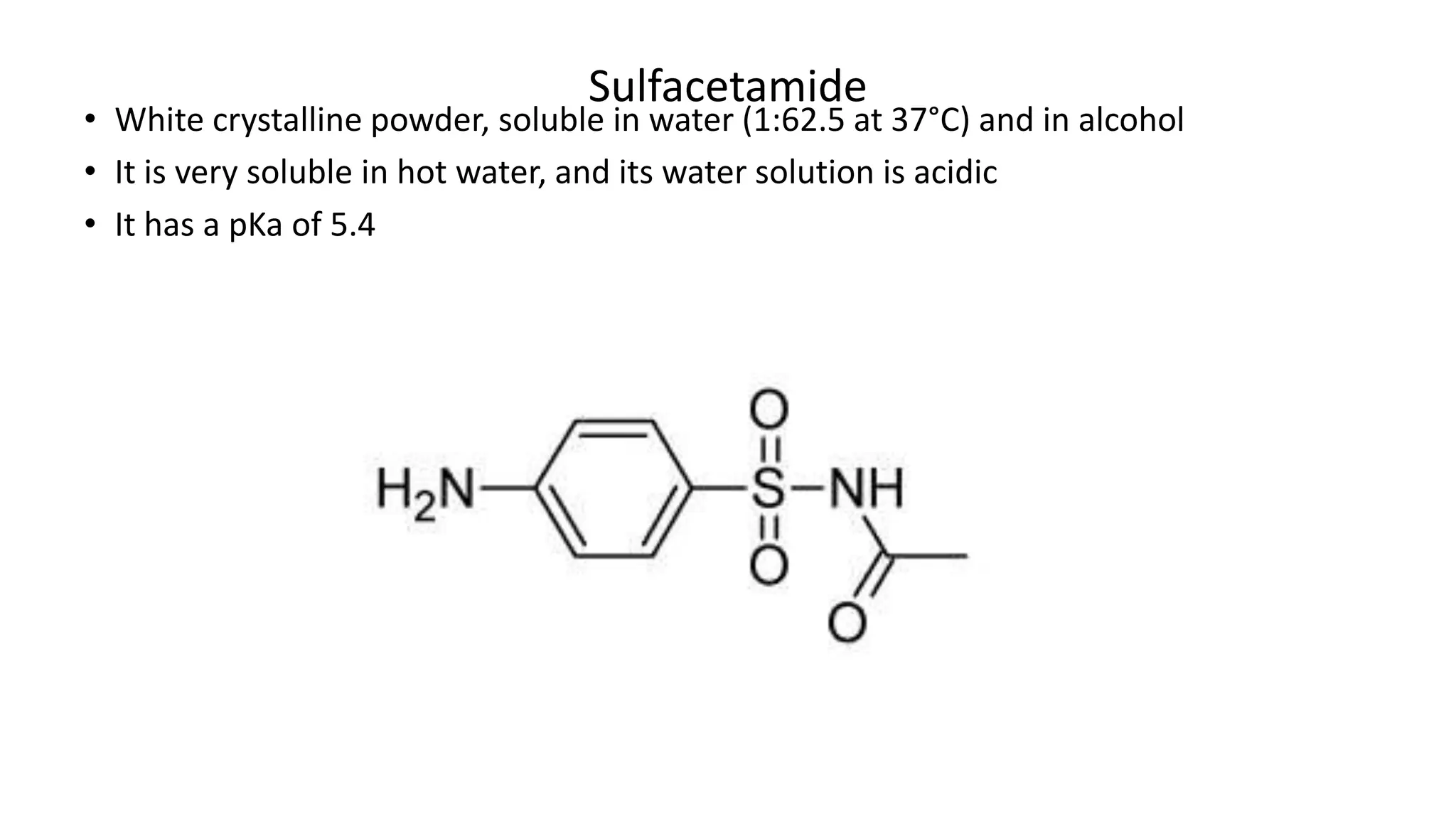
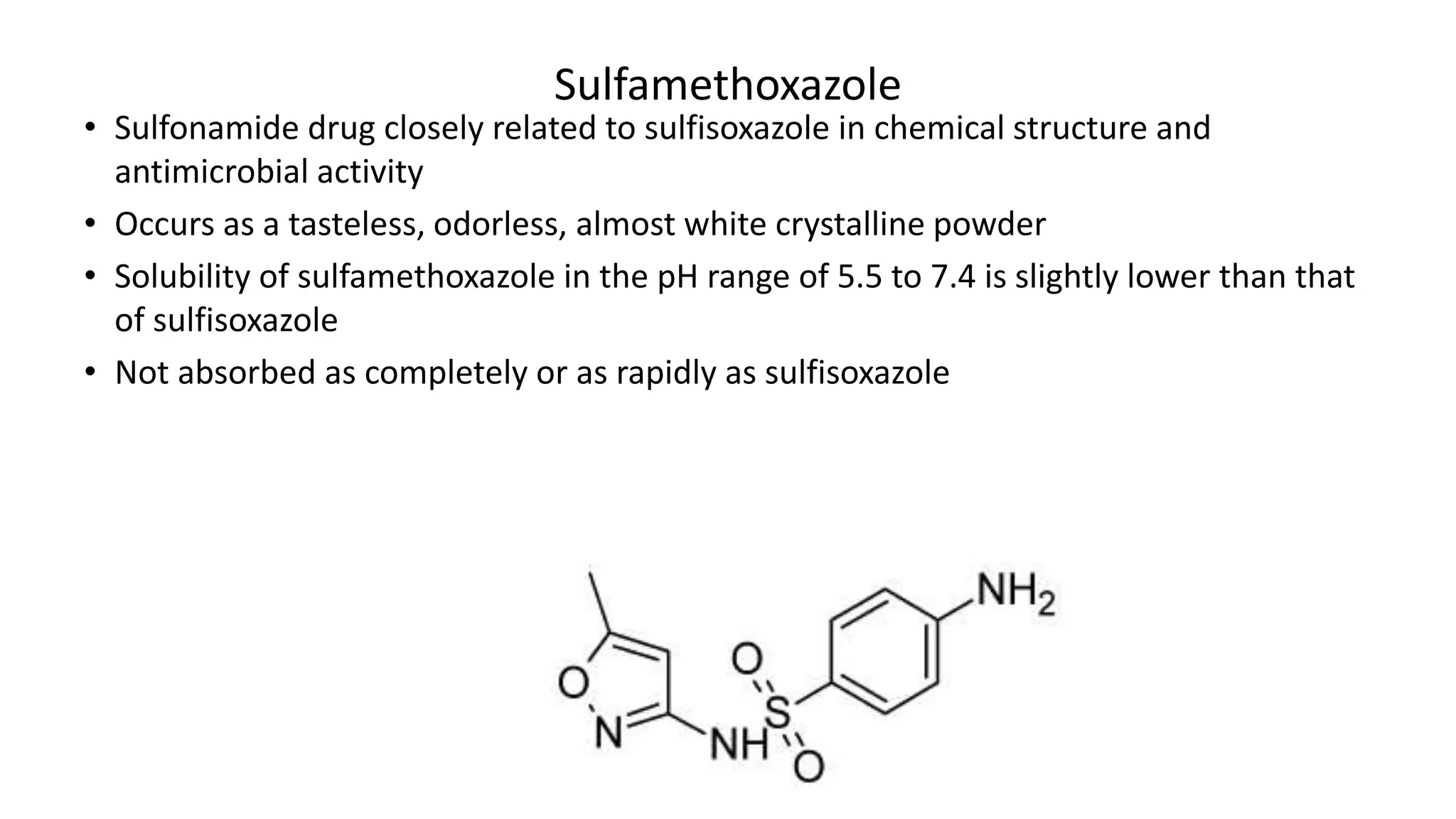
Sulphonamides are synthetic antimicrobial agents recognized for their bacteriostatic properties, primarily utilized to treat various bacterial infections, particularly urinary and respiratory tract infections. Although they were pivotal in the early days of antibiotics, increasing bacterial resistance and the emergence of alternative treatments have reduced their use in contemporary medicine. Despite these challenges, sulphonamides retain utility in specific applications, especially when combined with other agents, such as in the case of sulfamethoxazole and trimethoprim for pneumocystis pneumonia.





![Examples
Sulfadiazine: The systematic name is 4-amino-N-pyrimidin-2-ylbenzenesulfonamide.
Here, the sulfonamide is attached to a benzene ring which also contains a pyrimidin-
2-yl group and an amino group at positions 2 and 4, respectively.
Silver Sulfadiazine: Known chemically as silver 1-[(4-aminophenyl)sulfonyl]-2-
pyrimidinylazanide, indicating the presence of silver, a pyrimidinyl group, and a
sulfonamido linkage on an aniline (amino-benzene) ring.
Sulfonamide is a generic term that denotes three different cases:
1. Antibacterials that are aniline-substituted sulfonamides (the “sulfanilamides”)
2. Prodrugs that react to generate active sulfanilamides (i.e., sulfasalazine)
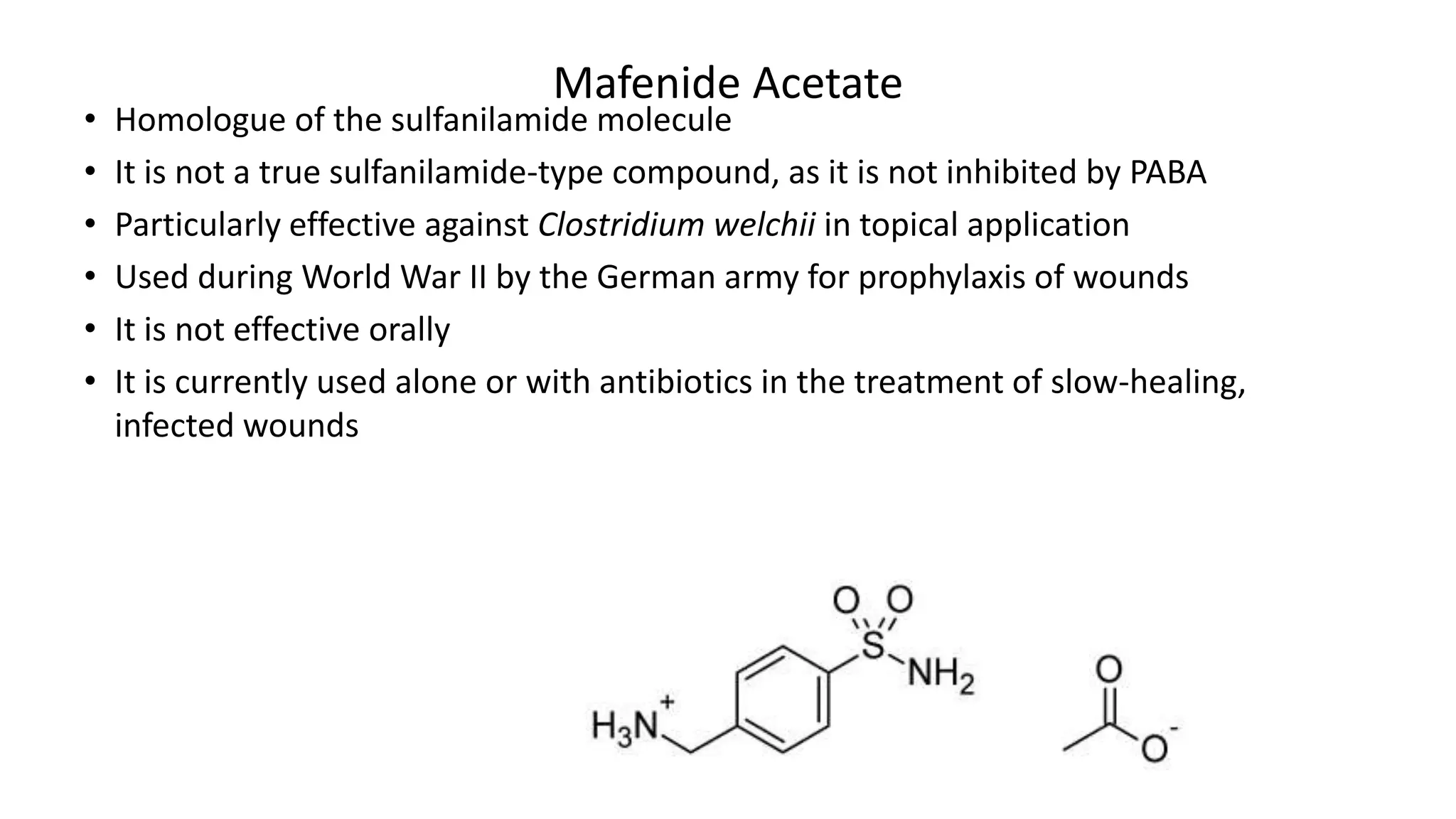
3. Nonaniline sulfonamides (i.e., mafenide acetate)](https://image.slidesharecdn.com/sulphonamides-240425154756-ec9af5a2/75/Sulphonamides-Medicinal-Chemistry-III-7-2048.jpg)