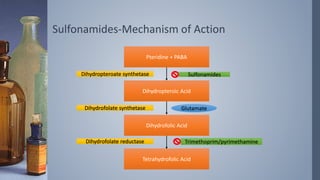
1. Sulfonamides and co-trimoxazole are antimicrobial agents that work by inhibiting folic acid synthesis in bacteria.
2. Co-trimoxazole is a synergistic combination of sulfamethoxazole and trimethoprim that blocks sequential steps in the folic acid pathway.
3. Common uses include urinary tract infections, respiratory infections, toxoplasmosis, and pneumocystis pneumonia prophylaxis in HIV/AIDS patients. Adverse effects include allergic reactions and crystalluria.








![Sulfonamides-Therapeutic Uses
› Due to development of high degree of resistance in large number
of bacteria systemic use of sulfonamides alone are seldom used.
› Therapeutic Uses:
– UTI:
› Co-trimoxazole is preferred agent.
› Sulfisoxazole 2-4g initial dose f/b 1-2g q6h for 5-10 days may be used for
uncomplicated cystitis.
– Nocardiosis:
› Sulfisoxazole/sulfadiazine 6-8g daily is alternative to Co-trimoxazole.
– Toxoplasmosis:
› Combination Sulfadiazine (1-1.5g PO q6h) + pyrimethamine (2g PO f/b 50-75mg PO
daily) + leucovorin (10-25 mg PO daily) for 6 weeks is combination of choice.
– Malaria:
› [Sulfadoxin (25mg/kg)+ Pyrimethamine (1.25mg/kg)] PO once on D1+ Artesunate
(4mg/kg/d) for 3 days for falciparum malaria.](https://image.slidesharecdn.com/sulfonamidesco-trimoxazole-200805043150/85/Sulfonamides-amp-co-trimoxazole-12-320.jpg)






![Co-trimoxazole- Therapeutic Uses
1. Urinary tract Infection
– Uncomplicated lower UTI
– Single dose of two DS tab. (800mg sulfamethoxazole+160mg Trimethoprim) OR one
DS tab twice daily for 3 days.
2. Bacterial Respiratory Tract Infection
– Acute exacerbation of chronic bronchitis
› 800-1200 mg sulfamethoxazole + 160-240 mg trimethoprim twice daily.
3. GI Infections
– Alternative to fluroquinolones for shigellosis.
4. Infection by pneumocystis jeroveci
– One DS tab once a day for prophylaxis in HIV-AIDS.
– High dose [15-20mg/kg/d trimethoprim + 75-100 mg/kg/d sulfamethoxazole] in 3-4
divided doses± glucocorticoids (Po2<70 mm Hg & A-a gradient <35mm Hg)](https://image.slidesharecdn.com/sulfonamidesco-trimoxazole-200805043150/85/Sulfonamides-amp-co-trimoxazole-19-320.jpg)


