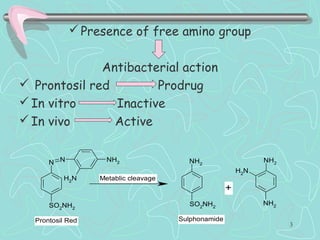
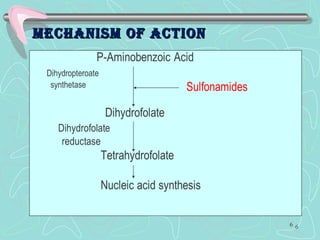
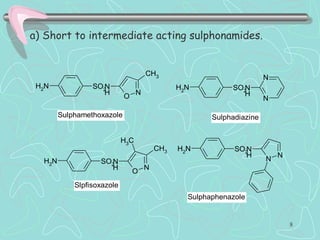
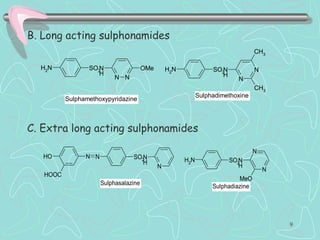
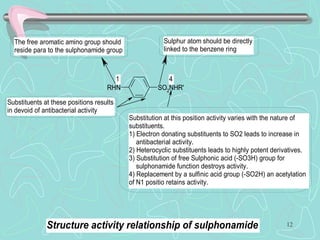
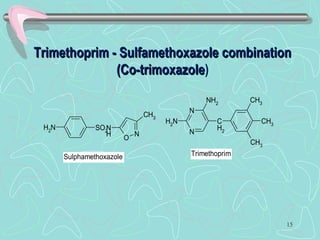
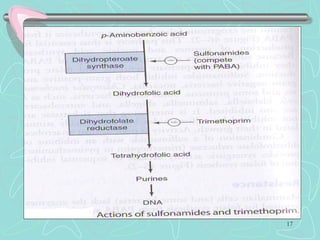
Sulphonamides are among the oldest antibacterial agents used to treat infections. They work by competitively inhibiting the enzyme dihydropteroate synthase, blocking folic acid synthesis in bacteria. This makes them bacteriostatic rather than bactericidal. Chemical modifications of the sulphonamide structure have led to important drug classes like diuretics, hypoglycemics, and anti-mycobacterials. Sulphonamides are commonly used to treat urinary tract, respiratory, and other bacterial infections. When combined with trimethoprim, they have broad-spectrum activity against many pathogens and are used as co-trimoxazole. Adverse effects can include allergic reactions and bone marrow suppression.