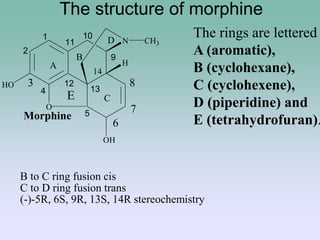
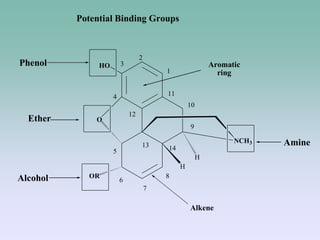
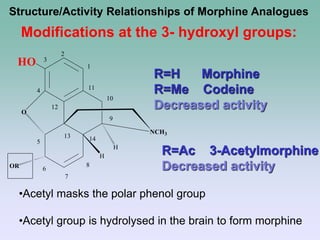
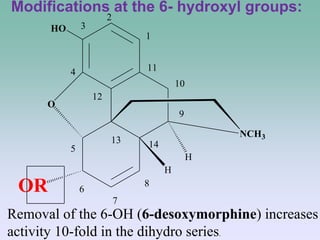
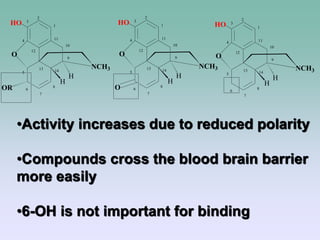
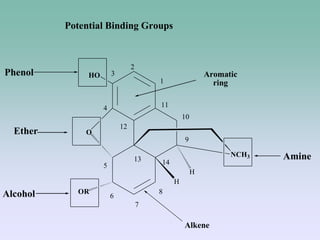
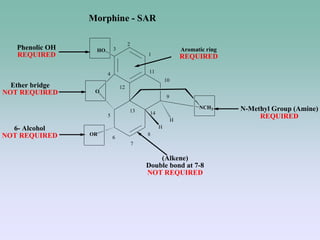
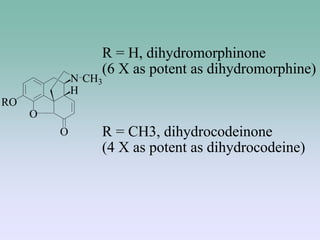
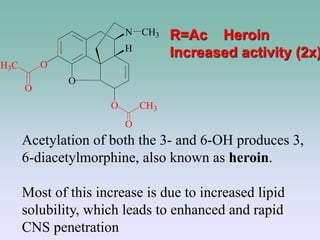
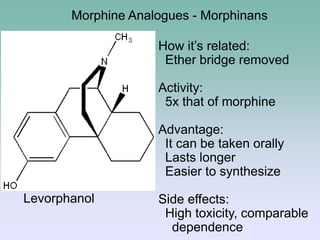
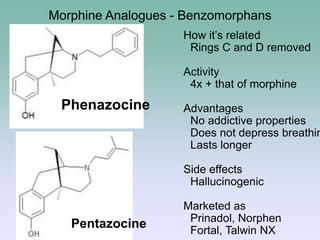
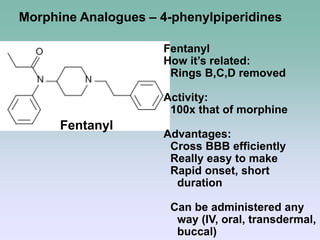
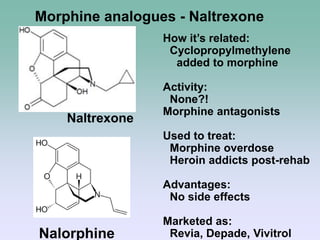
The document discusses the chemistry and structure-activity relationships of opioid analgesics, particularly focusing on morphine and its analogs. It details modifications to the morphine structure that affect potency and activity, such as changes at the hydroxyl groups and the introduction of acetyl groups, leading to compounds like heroin and other analogs. Various morphine derivatives are compared in terms of their effectiveness, side effects, and intended medical uses, highlighting the importance of molecular structure in opioid efficacy and safety.