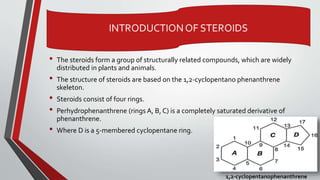
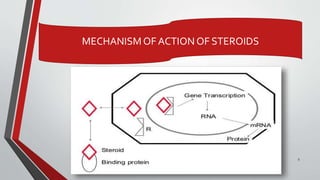
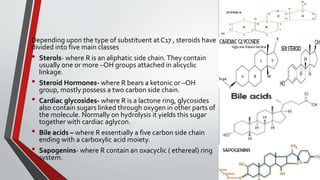
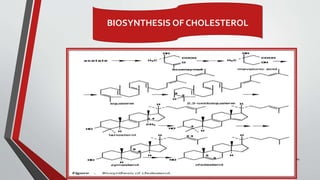
The document provides an overview of steroids, detailing their structure, classification, and mechanisms of action. Steroids are grouped based on their site of release, therapeutic class, and the type of substituent group attached, with specific categories like glucocorticoids, mineralocorticoids, and sex hormones identified. It also discusses the biosynthesis of cholesterol and steroid hormones, including the physiological effects of key steroid hormones.