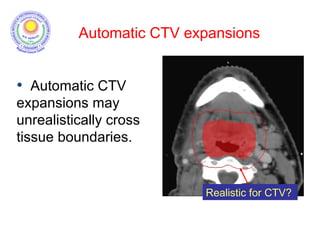
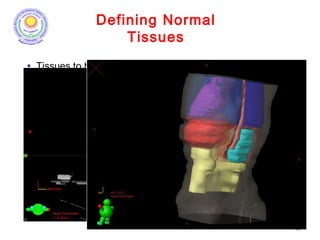
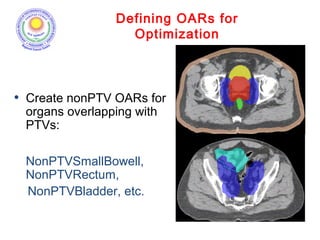
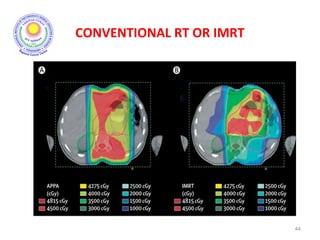
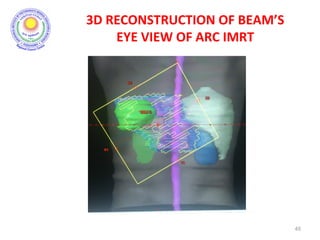
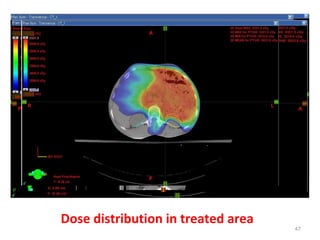
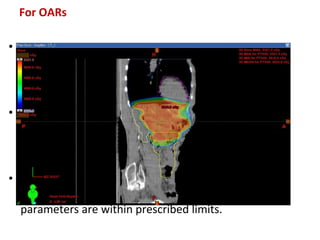
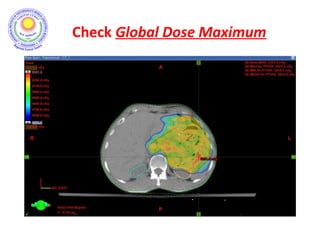
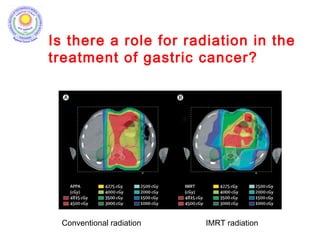
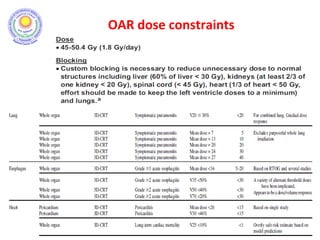
The document discusses adjuvant radiotherapy for locally advanced stomach cancers. It provides background on stomach cancer incidence and need for adjuvant therapy after surgery. Guidelines recommend adjuvant chemoradiation with 45Gy after surgery for advanced or node-positive disease. Target volumes include the stomach bed and regional lymph nodes. Organs at risk include kidneys, liver, lungs and spinal cord. Intensity modulated radiotherapy planning aims to meet dose constraints for targets and organs at risk.

![BACKGROUND…….
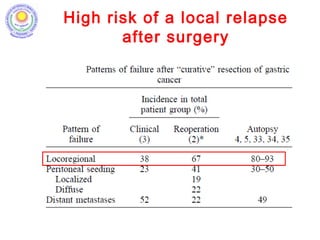
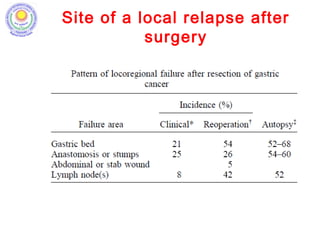
• Stomach cancer 5th most common cancer in males and
7th most common cancer in females in India. [V Rao DN,
Ganesh B. Estimate of cancer incidence in India in 1991. Indian J
Cancer, 1998]
• Surgery is standard of treatment; high incidence of
local and distal recurrence seen even after adequate
radical surgery; hence need for adjuvant therapy.
[D’Souza MA, Singh K, Shrikhande SV. J Cancer Res Ther 2009]
2](https://image.slidesharecdn.com/stomachadjuvantrt-170703072121/85/Stomach-adjuvant-rt-2-320.jpg)

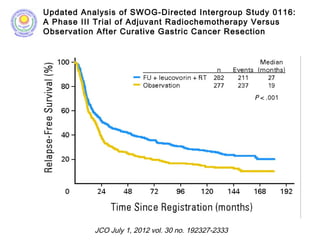
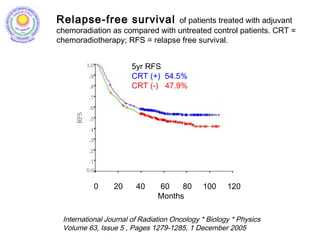
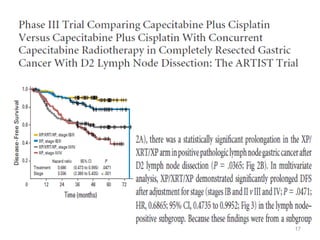
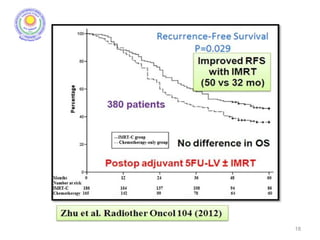
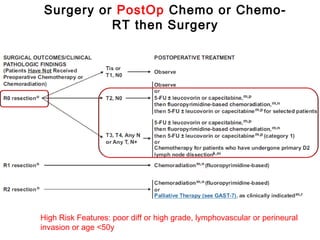
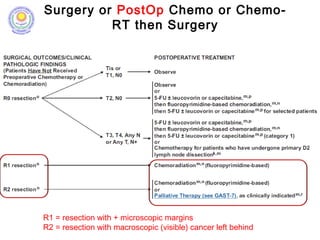
![• Post – operative chemo-radiation has been studied in the
INT-0116 Trial (MacDonald’s Trial)
[MacDonald JS et al. Chemoradiotherapy after surgery compared
with surgery alone for adenocarcinoma of the stomach or gastro-
esophageal junction. N Eng J Med 2001]
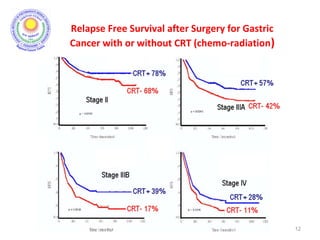
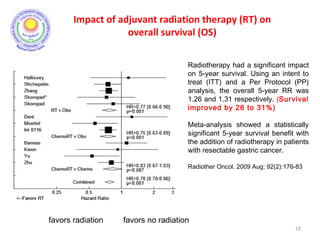
• There was significant improvement in survival and
decrease in local recurrence. BUT MORE THAN A THIRD
HAD GRADE 3 & 4 TOXICITIES.
9](https://image.slidesharecdn.com/stomachadjuvantrt-170703072121/85/Stomach-adjuvant-rt-9-320.jpg)


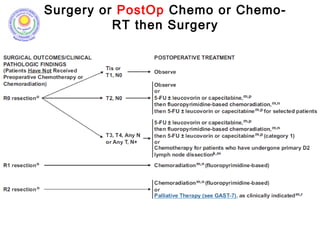
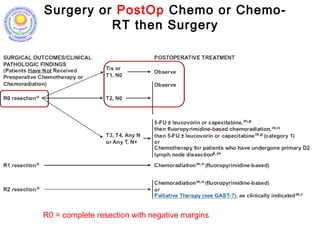

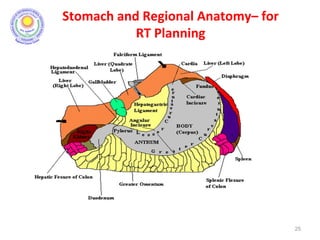
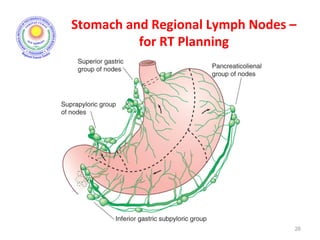
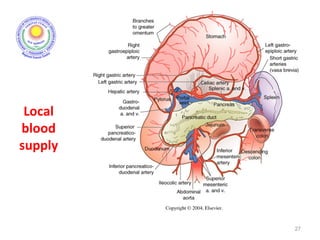
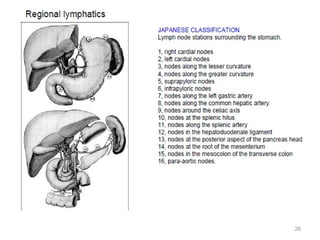

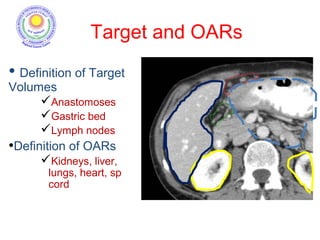
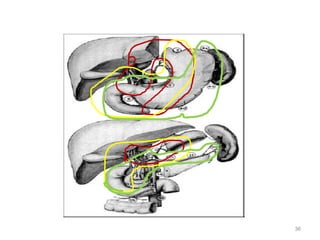
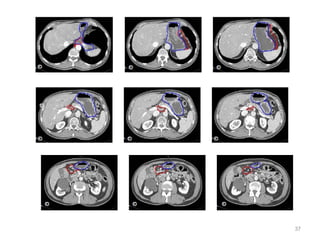
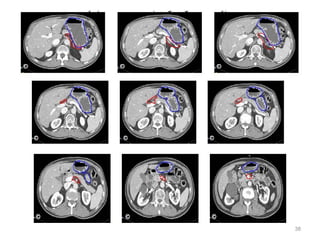
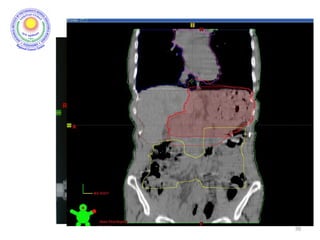
![Which lymph nodes have to be included in the CTV?
• individualize for GE-junction/Cardia (proximal), Corpus (middle)
and antrum (distal) tumors
• GE-junction/Cardia/proximal 1/3: para-oesophageal, perigastric,
hepatogastro lig, perigastric, ,celiac (left gastric artery, celiac
axis), splenic hilum, suprapancreatic, porta hepatis,
pancreaticoduodenal [stations 1-4;7,9-13]
• Corpus/middle 1/3: perigastric, suprapyloric, infrapyloric, celiac
(left gastric artery, common hepatic artery and celiac axis),
splenic hilum, suprapancreatic, porta hepatis,
pancreaticoduodenal [stations 3-13]
• Antrum/distal 1/3: perigastric, suprapyloric, infrapyloric, splenic
artery, pancreaticoduodenal, porta hepatis, celiac (left gastric
artery, common hepatic artery and celiac axis), suprapancreatic
[stations 3-9;11-13] 35](https://image.slidesharecdn.com/stomachadjuvantrt-170703072121/85/Stomach-adjuvant-rt-35-320.jpg)