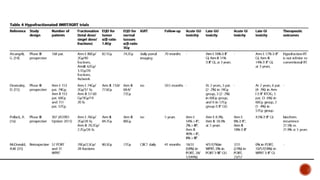
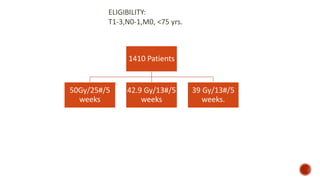
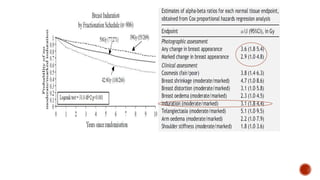
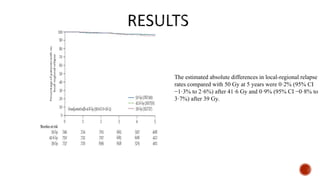
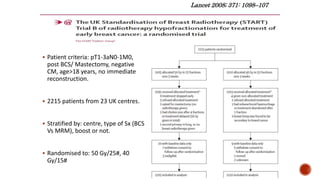
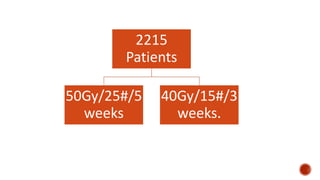
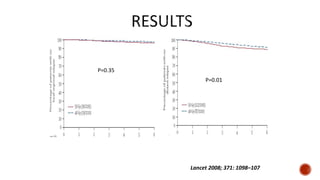
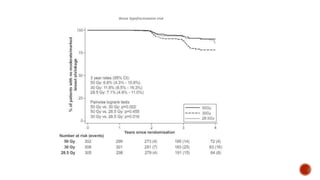
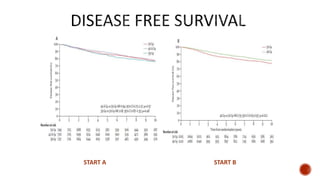
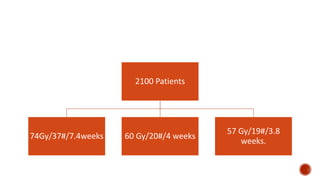
Hypofractionated radiotherapy regimens are being re-explored for their potential logistical benefits compared to conventionally fractionated radiotherapy. Several studies have evaluated hypofractionation for prostate cancer, finding comparable rates of tumor control and acceptable toxicity profiles. The CHHiP trial directly compared 57Gy in 19 fractions to 74Gy in 37 fractions for prostate cancer, finding no significant differences in patient-reported bowel symptoms up to 2 years post-treatment.

































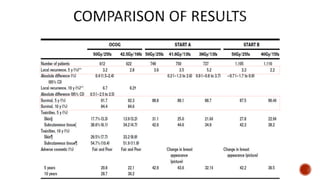
















![median follow-up was 60 months
The incidence of grade 2 or worse genitourinary toxicity at 3 years was 39·0%
(95% CI 34·2-44·1) in the standard fractionation group and 41·3% (36·6-46·4) in
the hypofractionation group
The incidence of grade 2 or worse gastrointestinal toxicity at 3 years was 17·7%
(14·1-21·9) in standard fractionation and 21·9% (18·1-26·4) hypofractionation.
Cumulative grade 3 or worse late genitourinary toxicity was significantly higher
in the hypofractionation group than in the standard fractionation group (19·0%
[95% CI 15·2-23·2] vs 12·9% [9·7-16·7], p=0·021
no significant difference between cumulative grade 3 or worse late
gastrointestinal toxicity (2·6% [95% CI 1·2-4·7]) in the standard fractionation
group and 3·3% [1·7-5·6] in the hypofractionation group; p=0·55).](https://image.slidesharecdn.com/hypofractionation-161217162518/85/HYPOFRACTIONATION-IN-RADIOTHERAPY-63-320.jpg)