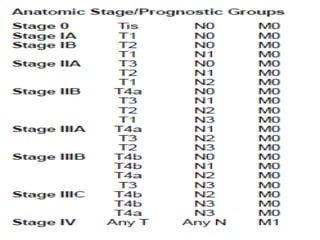
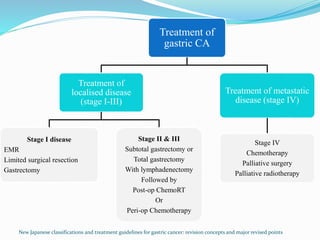
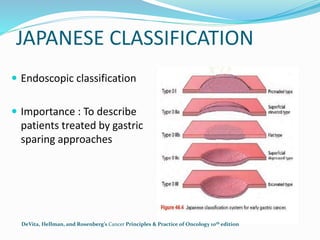
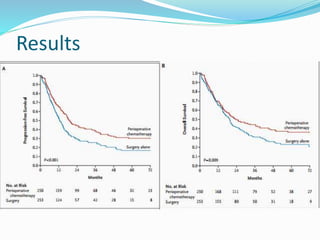
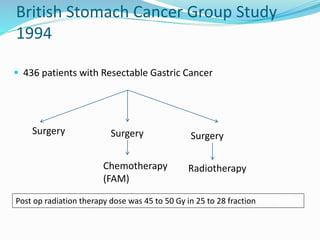
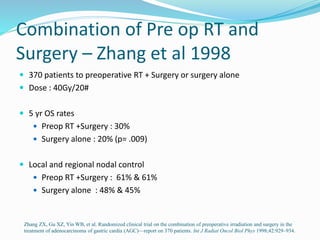
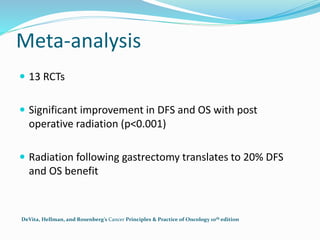
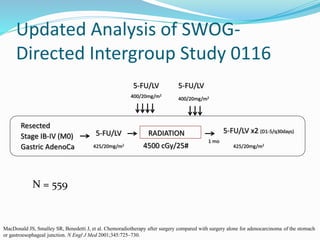
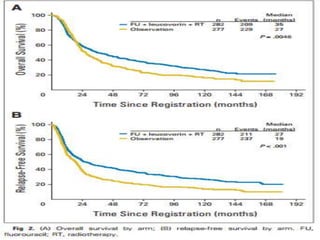
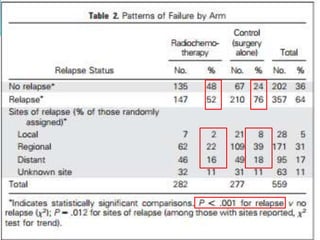
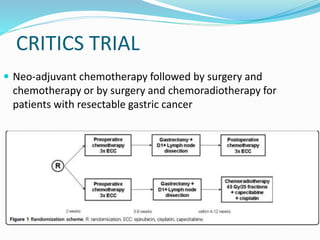
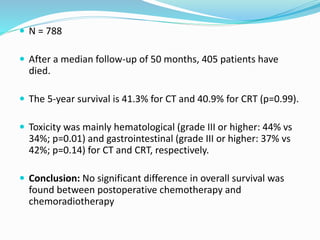
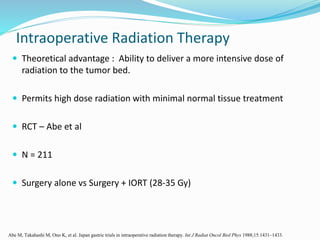
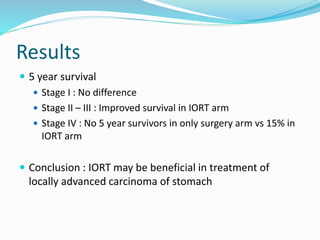
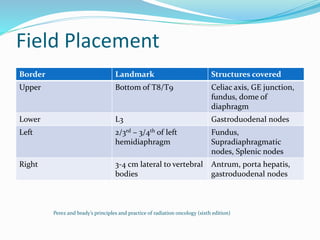
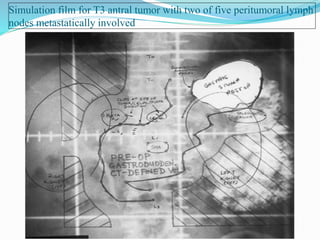
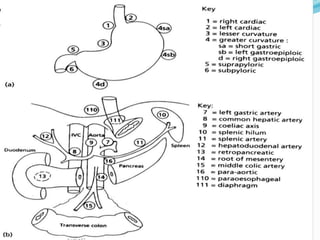
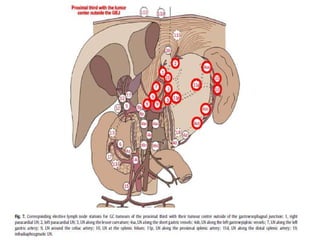
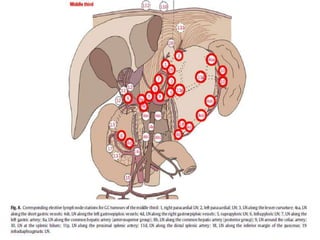
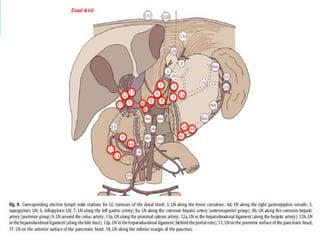
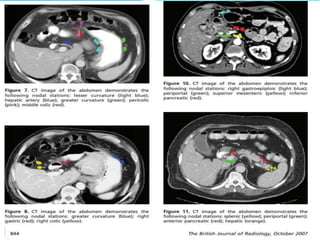
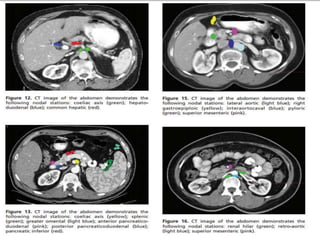
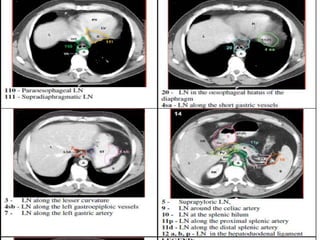
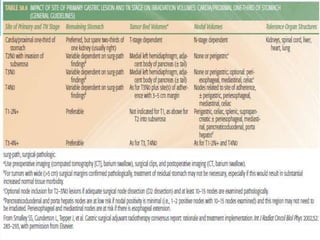
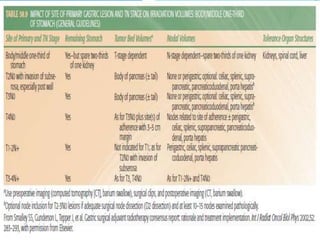
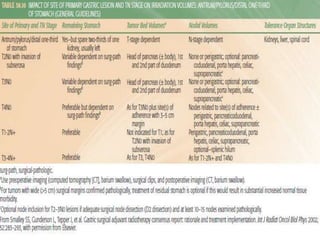
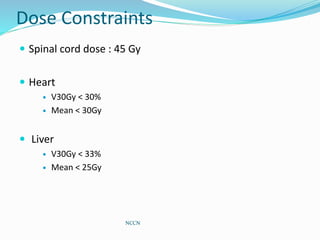
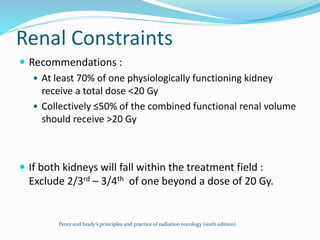
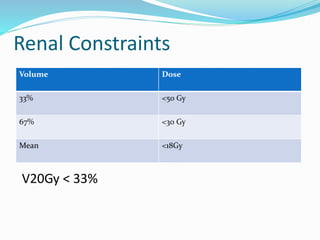
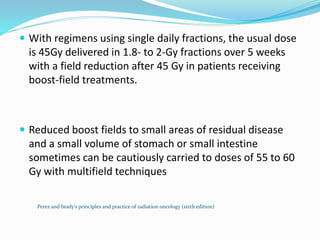
This document discusses treatment guidelines for gastric cancer. For localized disease, treatment may include endoscopic mucosal resection, limited surgical resection, or gastrectomy with lymph node dissection, followed by chemotherapy or chemoradiation. For metastatic disease, treatment includes chemotherapy, palliative surgery, or radiotherapy. Surgical techniques like subtotal or total gastrectomy with lymphadenectomy are described. The role of adjuvant and neoadjuvant chemotherapy and chemoradiation is also discussed. Simulation, target volumes, and dose constraints for radiation therapy are summarized.