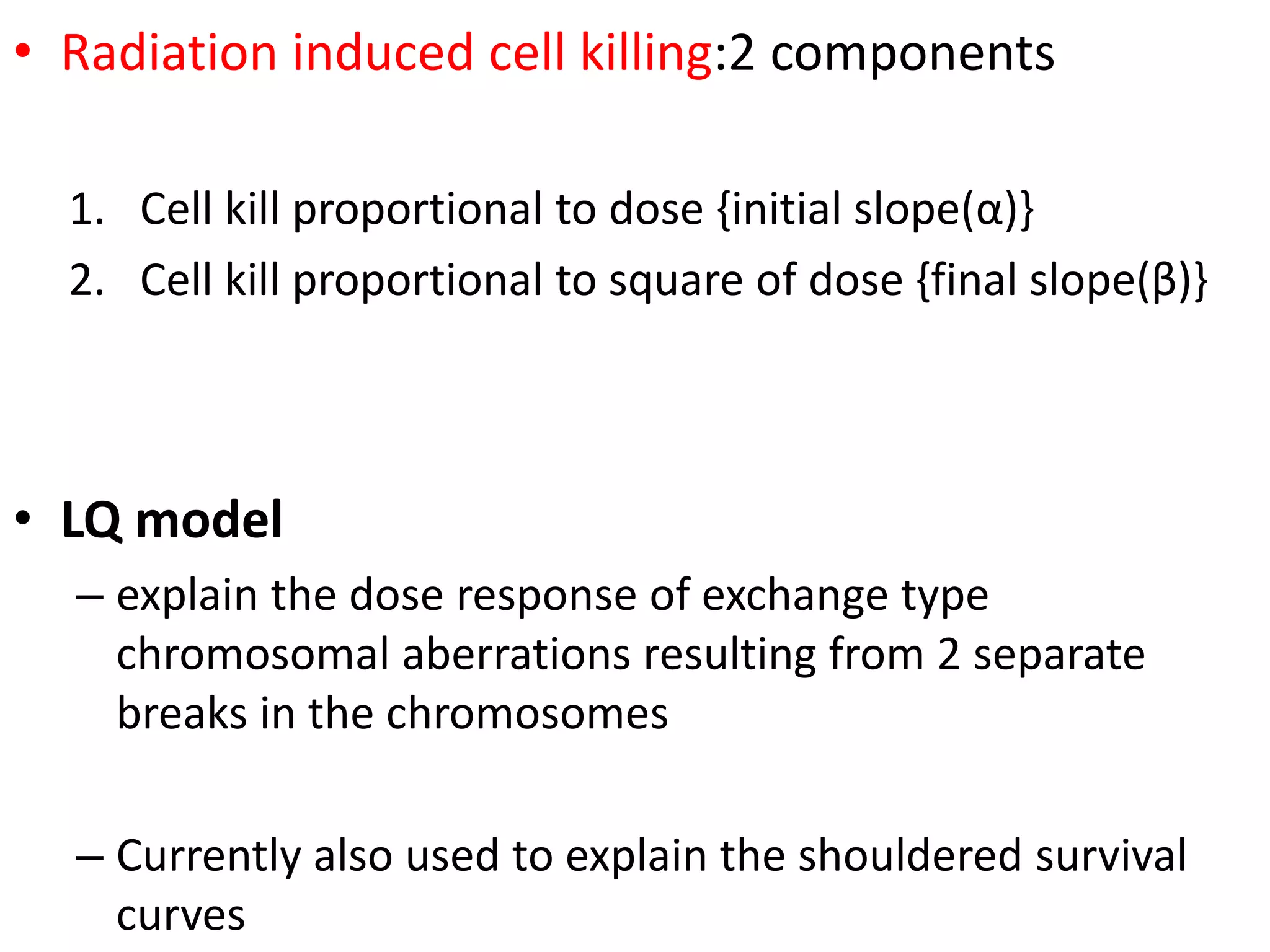
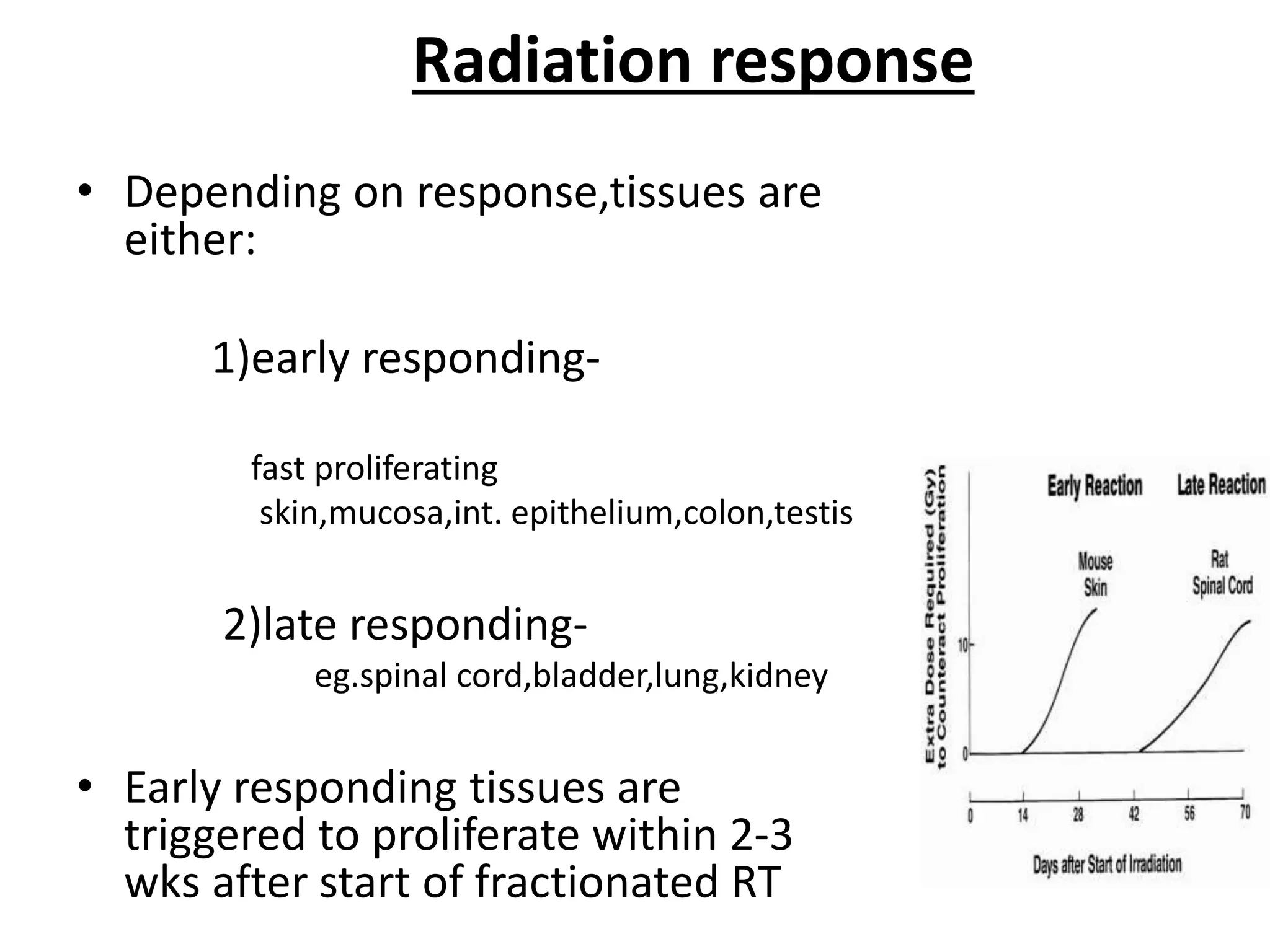
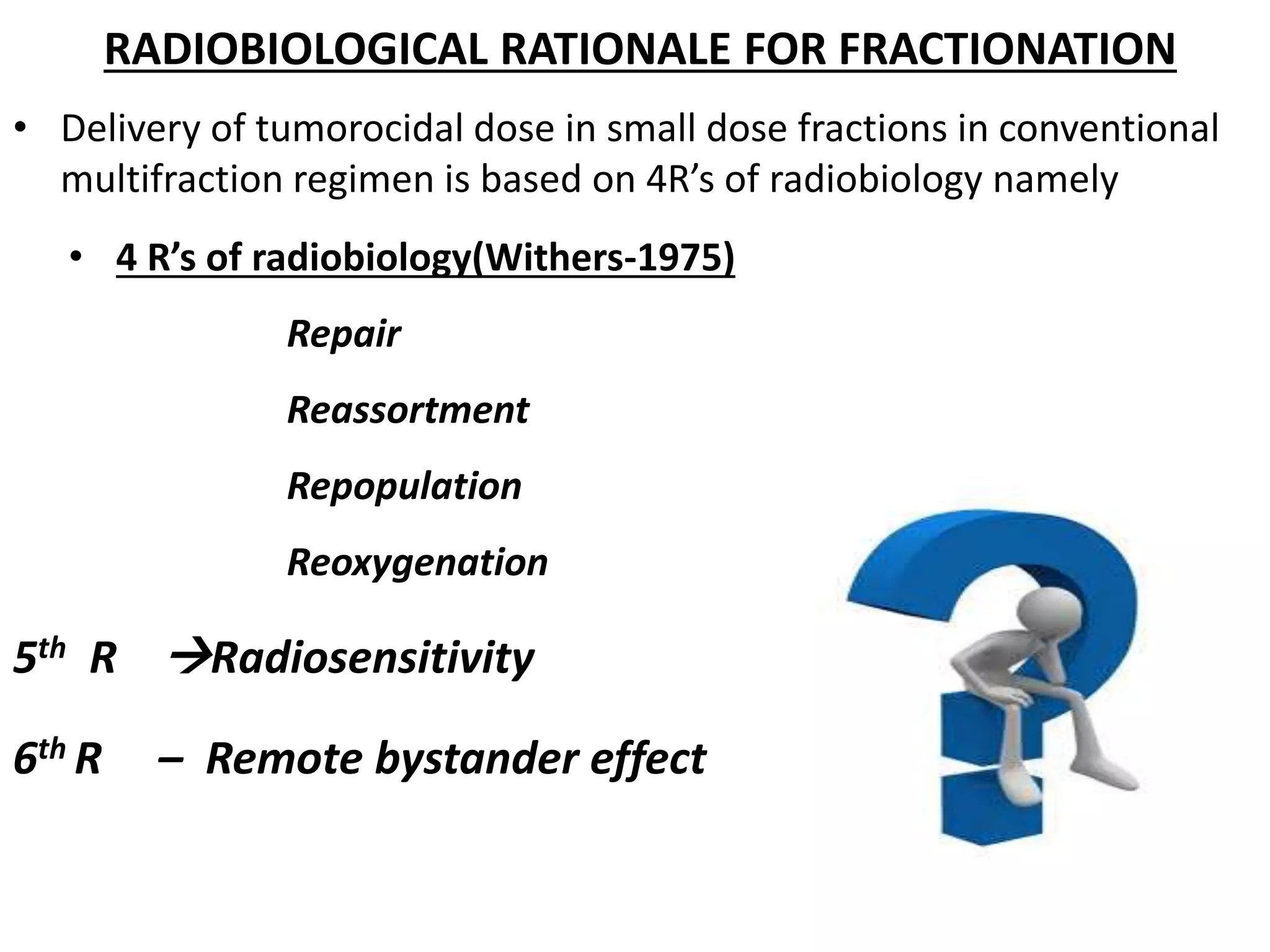
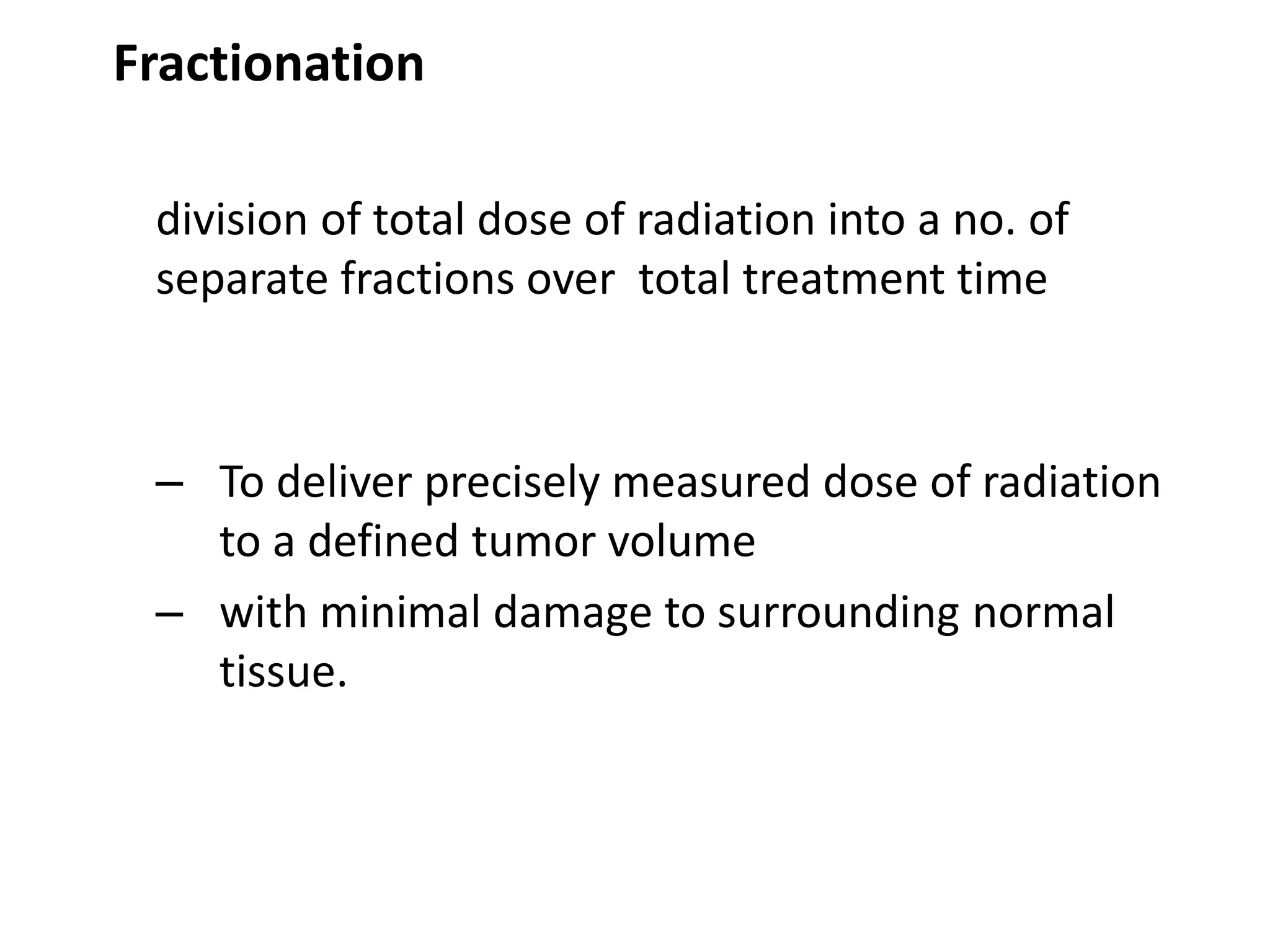
Fractionation involves dividing the total radiation dose into smaller daily fractions over the treatment period. This allows normal tissues to repair sublethal damage between fractions better than tumor tissues. Historical models like Stranquist cube root and Cohen's models related total dose and treatment time to biological effects. The linear quadratic model explains cell survival based on linear and quadratic components of radiation damage. Fractionation takes advantage of the four R's of radiobiology: repair of sublethal damage, repopulation, reoxygenation, and redistribution to improve the therapeutic ratio and deliver tumoricidal doses with minimal damage to surrounding normal tissues.


















![• TDF factor is derived from the basic NSD equation.
• TDF tables are available for rapid solution of NSD
problem.
• In split course regimes, overall effect= sum of TDF
factors
• TDF1 . [T/T+R]0.11 + TDF2](https://image.slidesharecdn.com/fractionationinradiotherapy-200104164036/75/Fractionation-in-radiotherapy-23-2048.jpg)