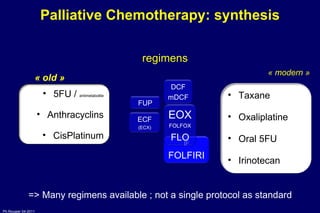
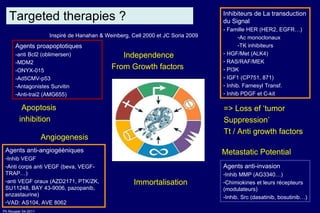
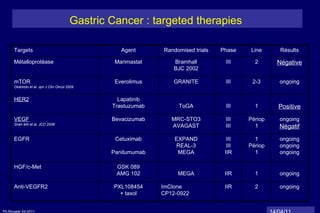
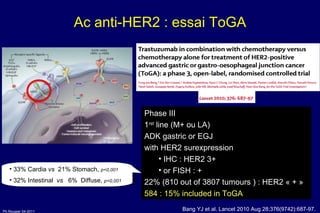
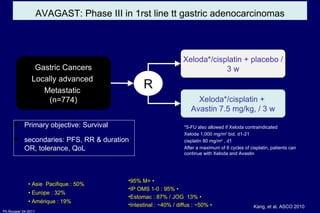
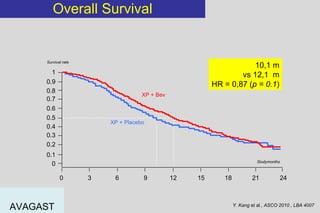
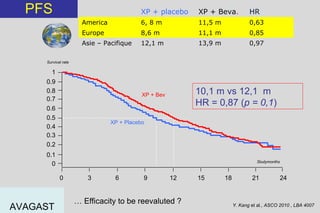
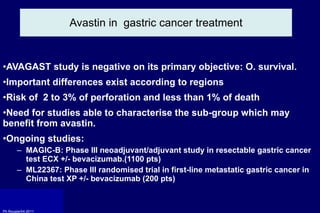
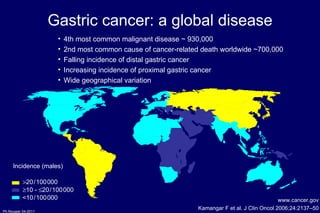
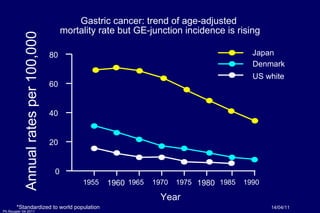
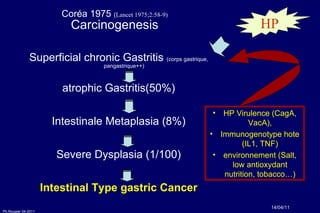
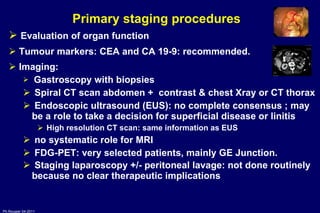
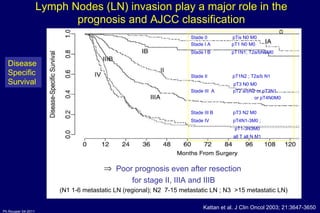
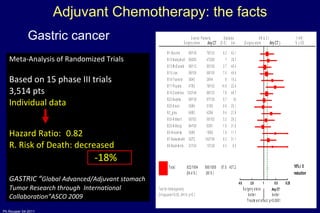
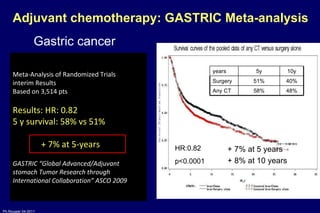
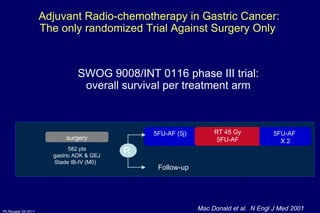
The document summarizes current standards and next steps in treating gastric cancer. It discusses trends showing falling incidence of distal gastric cancer but rising incidence of proximal gastric cancer. It reviews primary staging procedures and treatments for gastric cancer including surgery, adjuvant treatments, and treatments for advanced cases. It provides evidence that adjuvant chemotherapy and perioperative chemotherapy can increase overall survival rates compared to surgery alone.
![Gastric cancer: Current standards and next steps Philippe ROUGIER, Sce HGE-Oncologie Digestive HEGP, 75015 PARIS [email_address] UFR PIFO UVSQ Université Versailles-Saint Quentin en Yveline](https://image.slidesharecdn.com/20rougiergastric-110414152158-phpapp02/75/MON-2011-Slide-20-P-Rougier-Gastric-and-pancreatic-cancers-part-I-1-2048.jpg)






![S1 compound in adjuvant (2007) Benefit on DFS & OS at 3 year (+ 12% and + 10.4%) Benefit non dependant on age or sexe… mainly stage II and IIIa tumors 100 50 0 2 3 4 5 1 Ans HR = 0,68 [0,52-0,87] p = 0,0024 Overall survival (n = 1 059) 100 50 0 2 3 4 5 1 Ans HR = 0,62 [0,50-0,77] p < 0,0001 Disease free Survival (n = 1 059) % % ASCO GI 2007 – D’après Sasako et al., Tokyo, Japon, abstr. 8 actualisé ; lancet 80,5 % 70,1 % Surgery + S-1 Surgery only 72,2 % 60,1 % Surgery + S-1 Surgery only](https://image.slidesharecdn.com/20rougiergastric-110414152158-phpapp02/85/MON-2011-Slide-20-P-Rougier-Gastric-and-pancreatic-cancers-part-I-15-320.jpg)












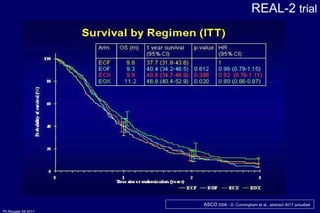

![FNLCC-GERCOR-FFCD 0307 FOLFIRI / ECX as first line CT : primary objective : Time to First line treatment Failure p (Log-rank) = 0.008 HR ( B vs A) = 0.77 [0.63;0.94] ECX 1 rst line : 4.24 m [3.48; 4.65] FOLFIRI 1 rst line : 5.09 m [4.53; 5.68] Overall Survival : ECX 1 ère ligne) : 9.49 m. [ 8.77; 11.14] FOLFIRI 1 ère ligne) : 9.72 m . [8.54; 11.27] p (Log-rank)= 0.95 HR (B vs A)= 1.01 [0.82; 1.24] less toxicity with FOLFIRI = Guimbaud R et al ASCO 2010 Bras A 209 108 33 8 4 2 1 1 1 Bras B 207 123 50 19 6 3 2 1 0 TTF 0.0 0.2 0.4 0.6 0.8 1.0 Time (months) 0 4 8 12 16 20 24 28 32](https://image.slidesharecdn.com/20rougiergastric-110414152158-phpapp02/85/MON-2011-Slide-20-P-Rougier-Gastric-and-pancreatic-cancers-part-I-46-320.jpg)