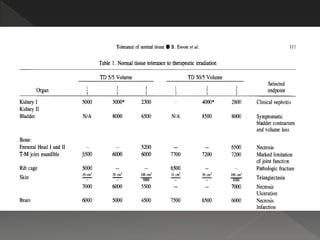
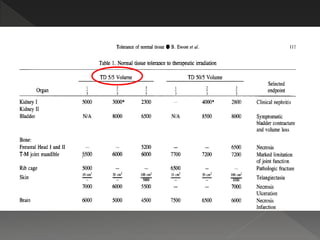
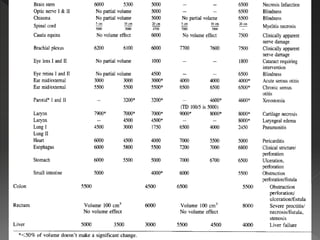
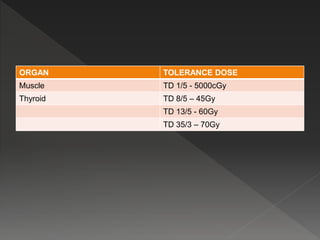
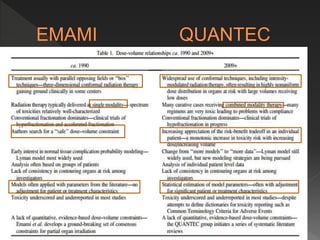
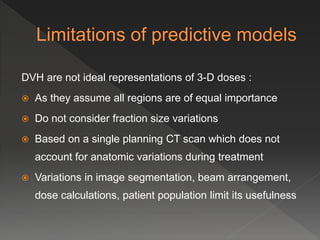
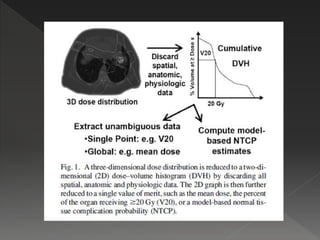
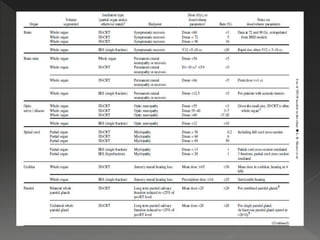
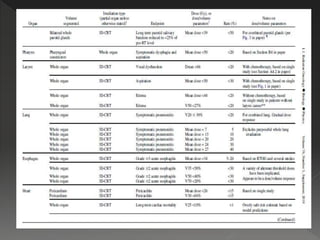
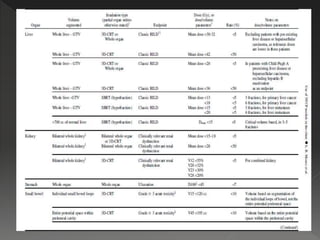
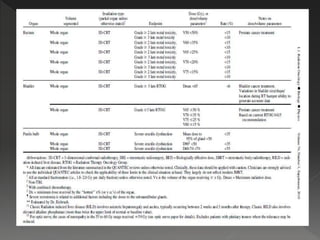
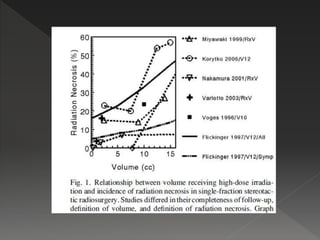
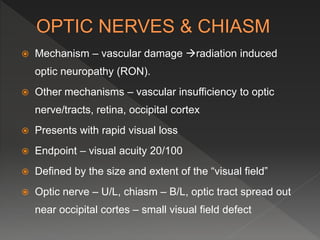
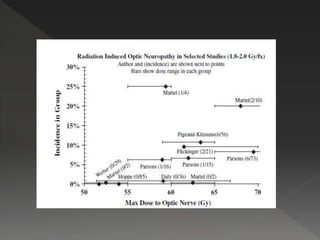
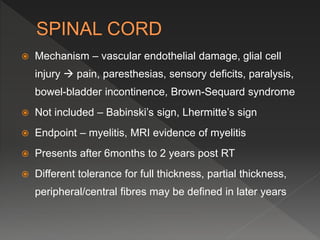
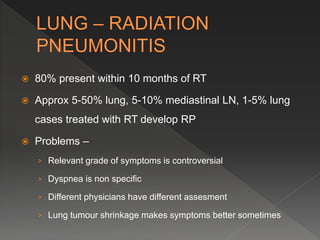
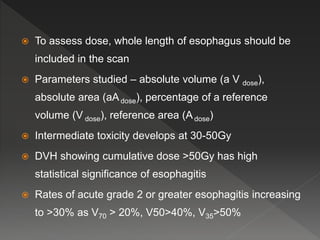
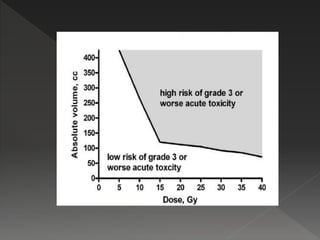
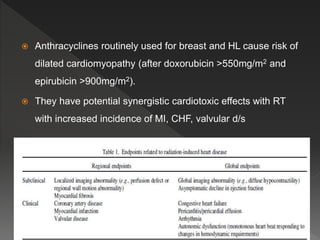
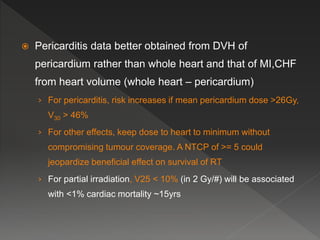
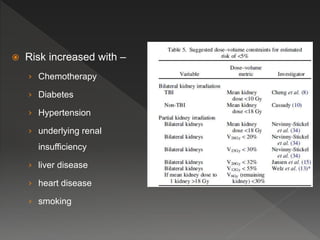
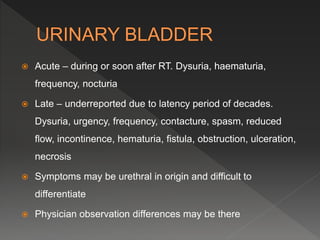
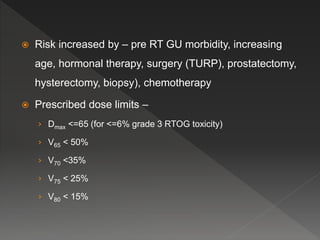
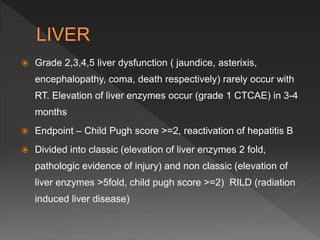
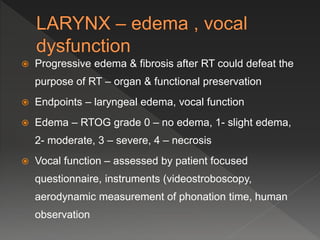
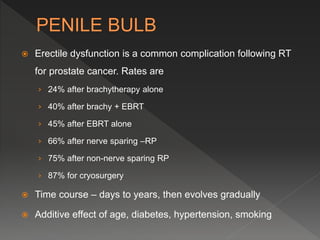
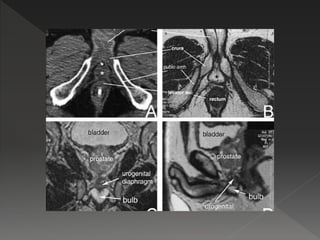
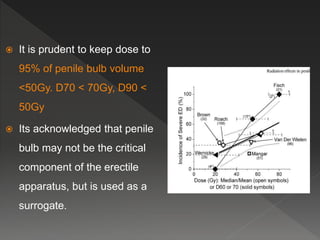
This document discusses normal tissue tolerance doses from radiation therapy. It describes the formation of a task force to establish tolerance protocols, with an emphasis on partial volume effects. The earliest publication of tolerance doses is cited from 1972. 28 critical organ sites were included and considered in terms of dose, time factors, and partial volumes irradiated. The significance of these parameters and a quantitative model for normal tissue complication probability are provided. Limitations of the available data and ongoing areas of research are also outlined.





![ The significant parameters considered were –
› Dose – time factors
› Partial volumes of normal tissues irradiated
Only the linear component of the linear quadrantic
model was used.
The expression of the NTCP model equation may be
written as :
NTCP (D,v) = exp [-N0 v-k exp {-aDG}]](https://image.slidesharecdn.com/quantec-dr-161024184901/85/Quantec-dr-upasna-saxena-2-7-320.jpg)
![ G = [1+d/(a/b)]
a is the coefficient of lethal damage
b/a is the reciprocal of a/b
N0 (depicts proportion of stem cells) and k (depicts volume
dependence) are the tissue/organ specific, non-negative
adjustable parameters
V is the uniformly irradiated partial volume of the tissue/organ
(=V/Vref where V is the uniformly irradiated volume of the
normal organ, Vref is the reference volume of the normal
organ.](https://image.slidesharecdn.com/quantec-dr-161024184901/85/Quantec-dr-upasna-saxena-2-8-320.jpg)