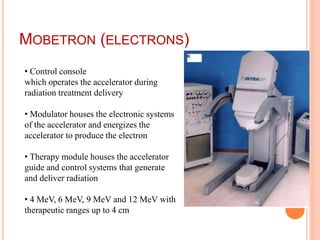
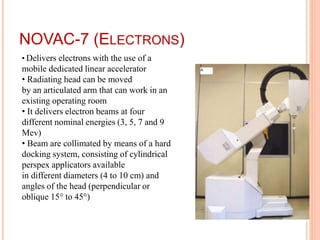
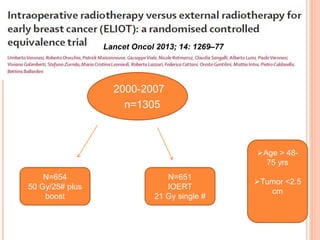
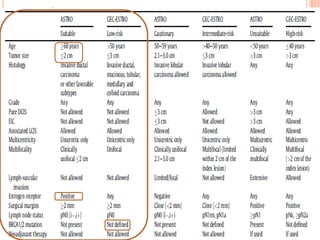
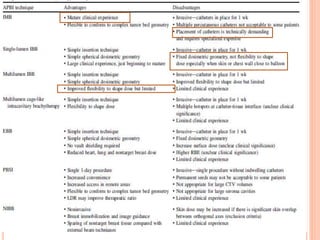
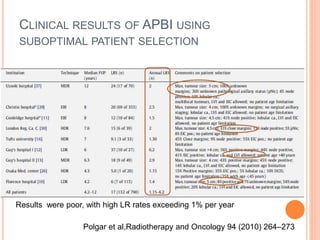
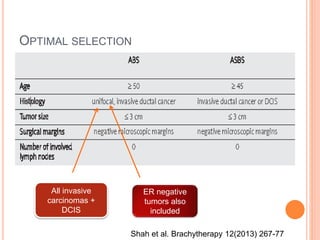
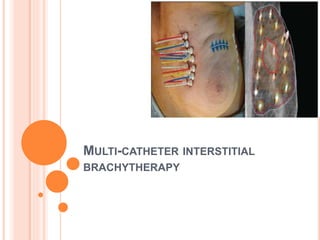
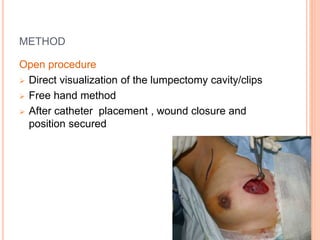
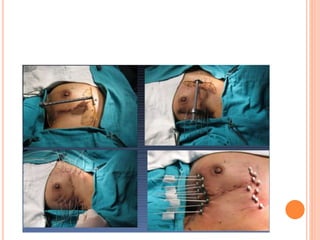
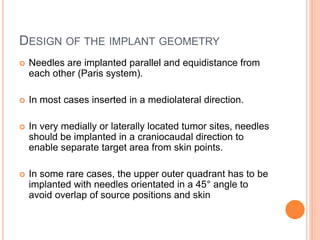
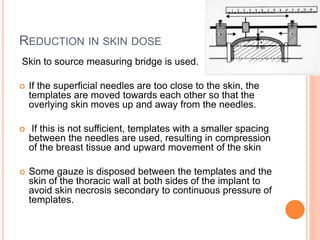
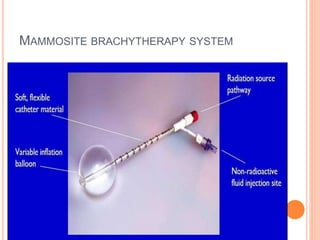
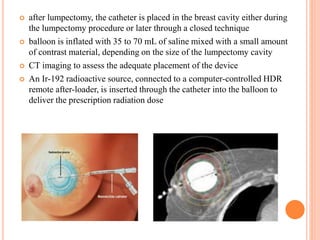
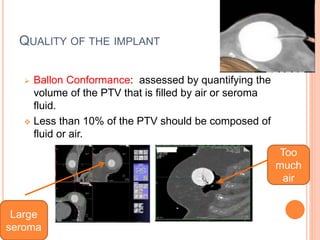
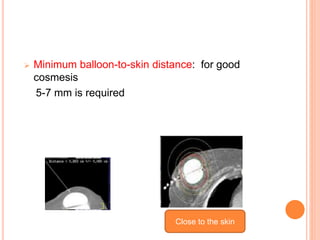
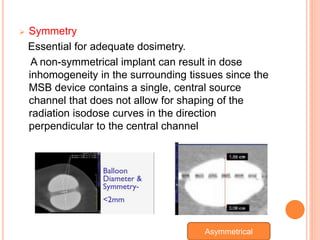
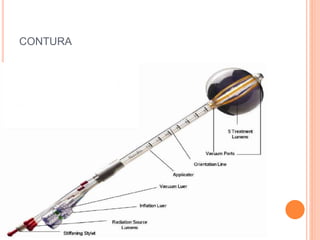
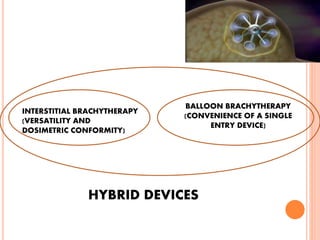
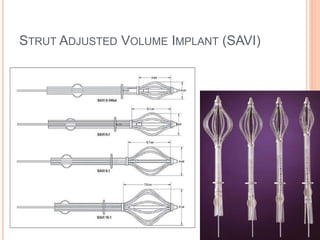
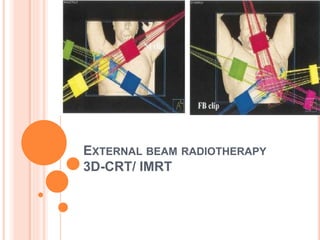
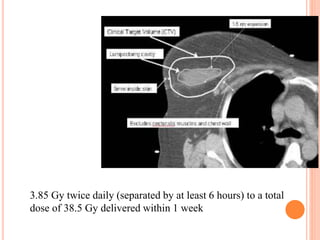
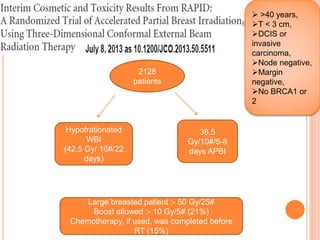
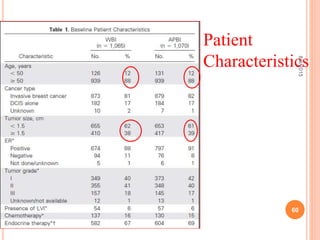
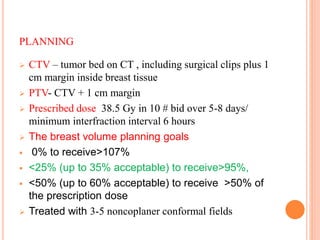
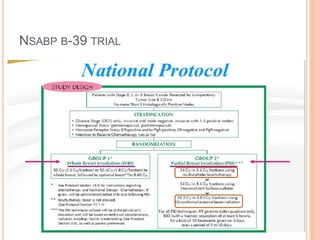
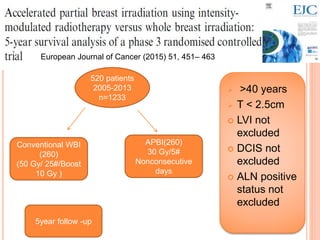
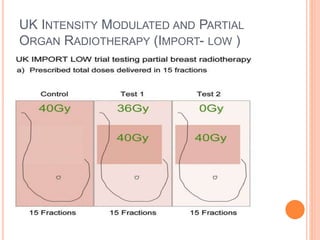
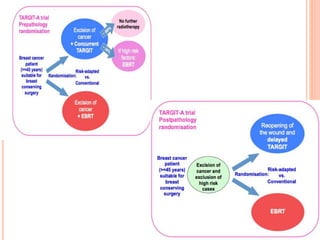
Accelerated partial breast irradiation (APBI) delivers radiation to only the portion of the breast at highest risk of recurrence rather than the whole breast. This allows radiation to be delivered in a significantly shortened period. Several techniques for APBI exist including brachytherapy using catheters implanted in the breast, balloon brachytherapy, and external beam radiotherapy. Ongoing clinical trials are evaluating outcomes and toxicities of APBI compared to whole breast irradiation in appropriately selected patients with early-stage breast cancer.




























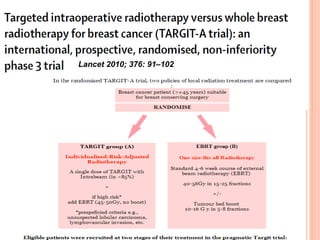
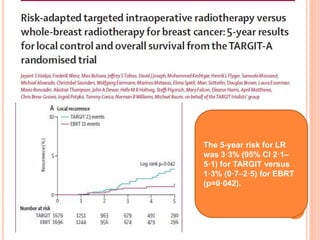
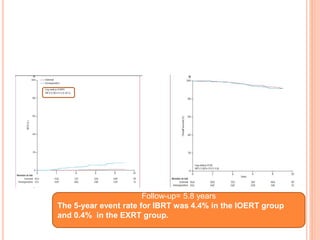
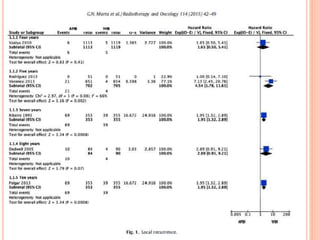
![P= 0.41(NS)
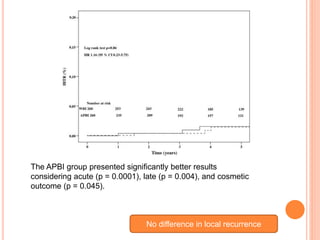
N0 difference in local recurrence
Radiotherapy toxicity was lower in the targeted intraoperative
radiotherapy group (six patients [0·5%]) than in the external beam
radiotherapy group (23 patients [2·1%]; p=0·002).](https://image.slidesharecdn.com/acceleratedpartialbreastirradiation-130905230123-/85/Accelerated-partial-breast-irradiation-73-320.jpg)