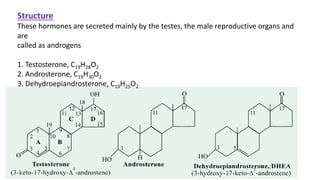
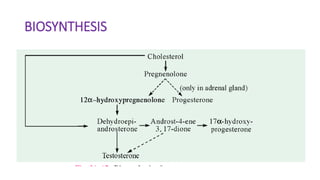
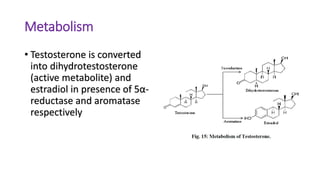
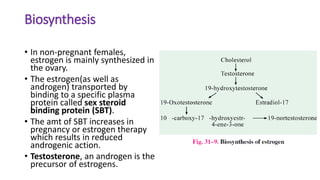
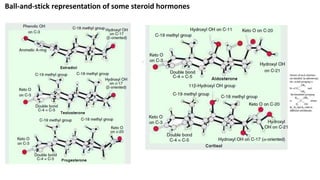
Sex hormones are steroid hormones that affect the growth and function of reproductive organs or development of secondary sex characteristics. They are classified into androgens, estrogens, and gestogens. Androgens like testosterone are male hormones mainly produced in testes. Estrogens like estrone and estradiol are female hormones produced in ovaries. Gestogen progesterone is produced in the corpus luteum and placenta during pregnancy. These hormones have important roles in sexual development and reproductive functions.