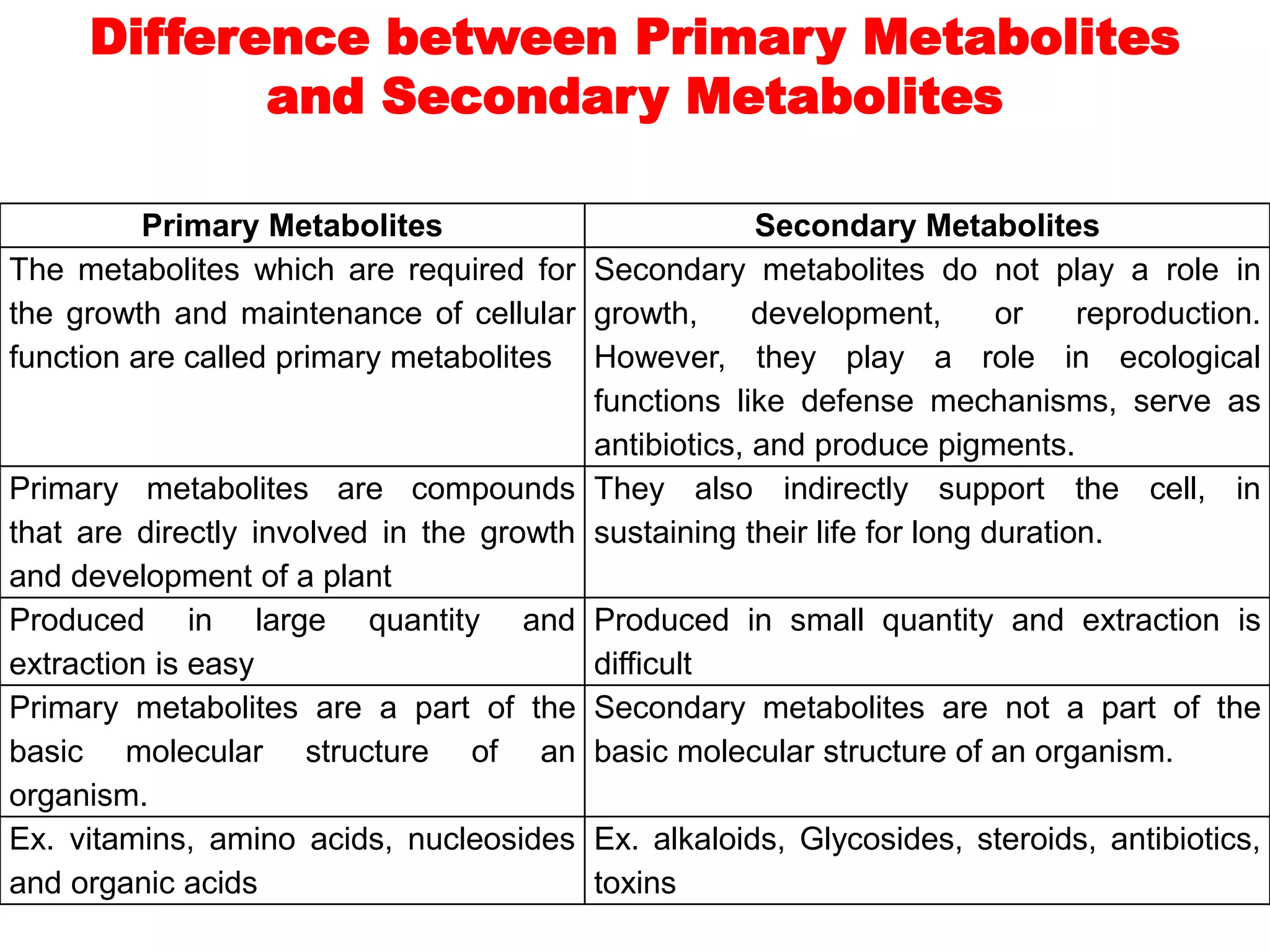
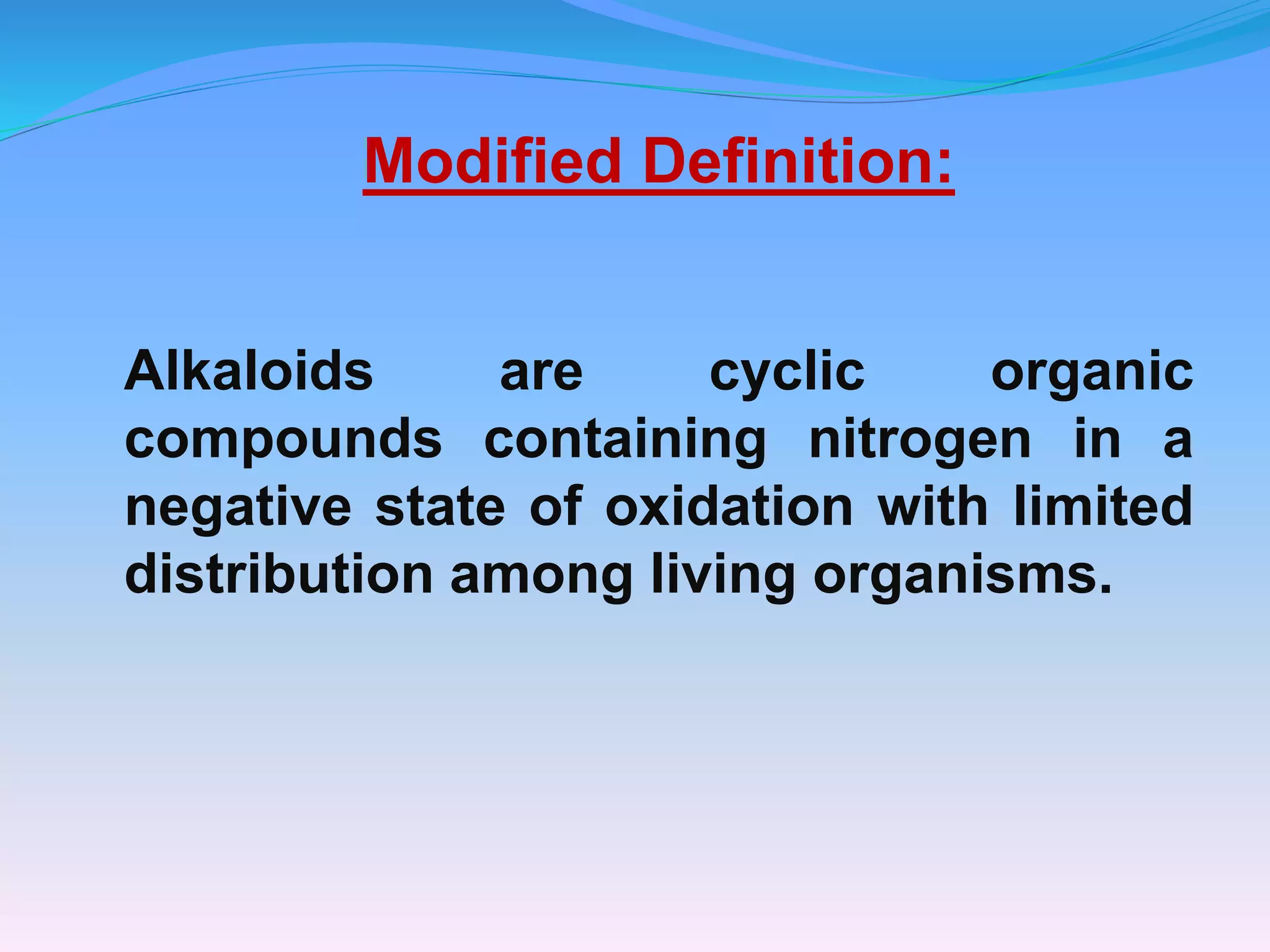
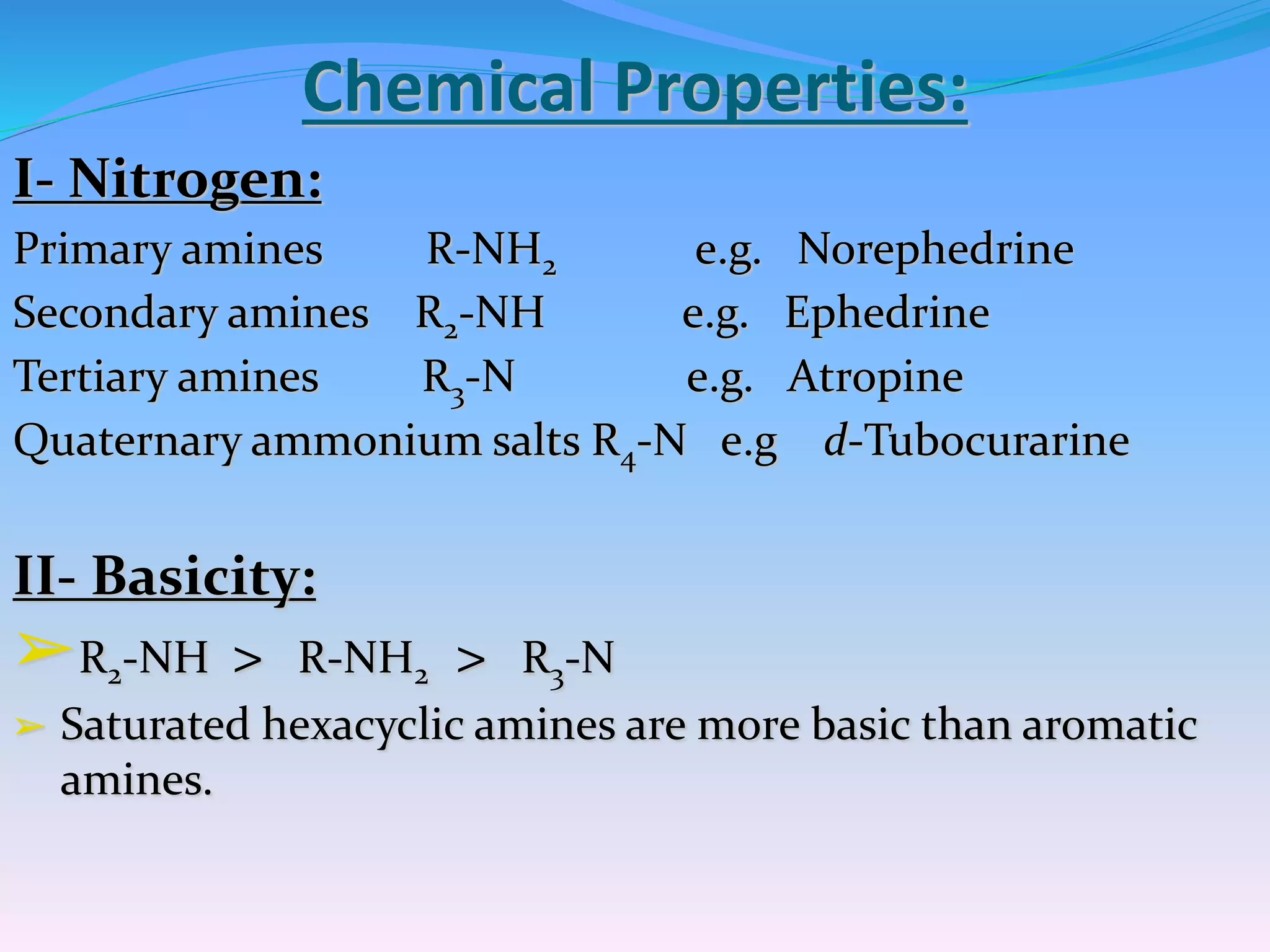
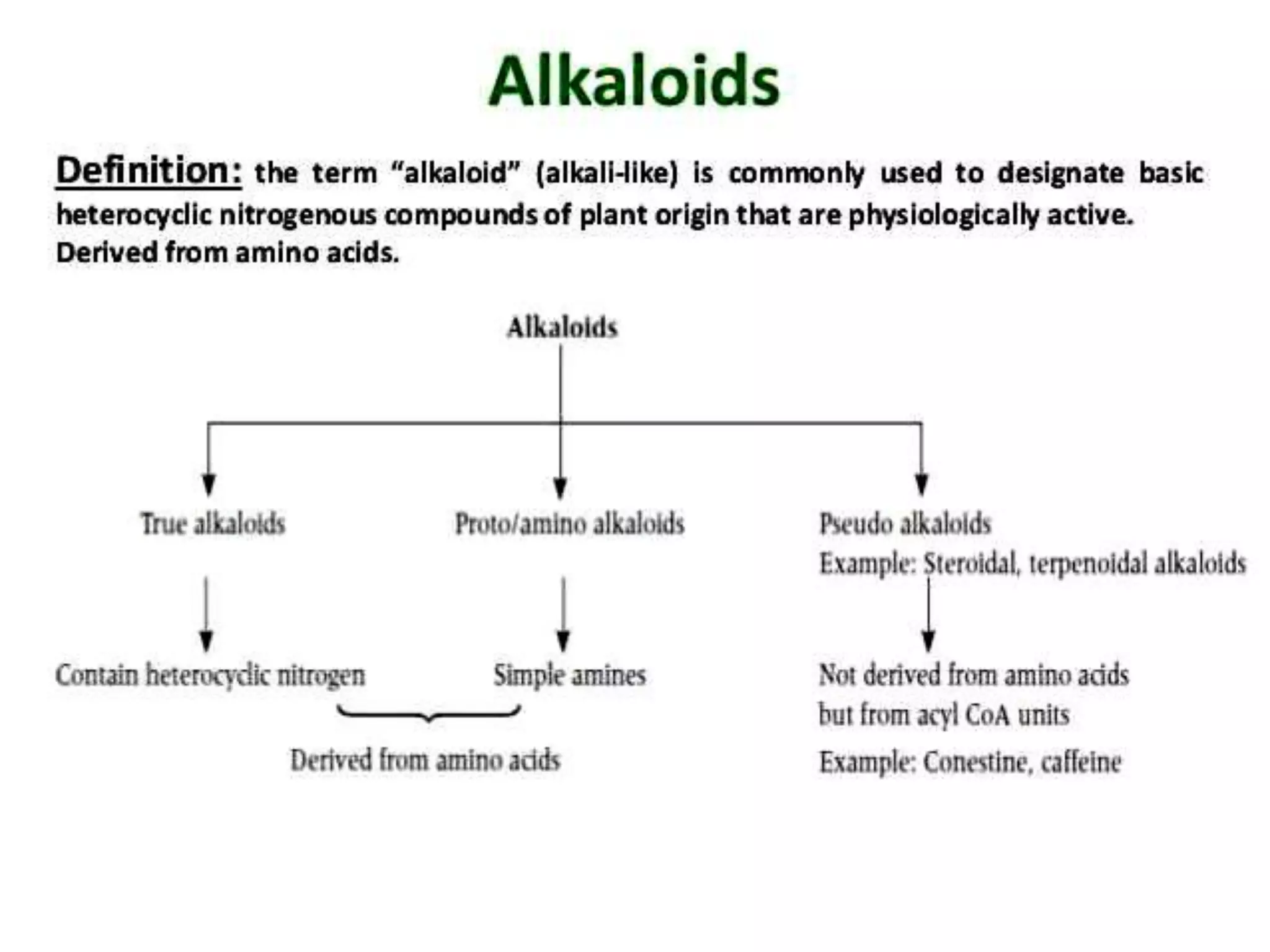
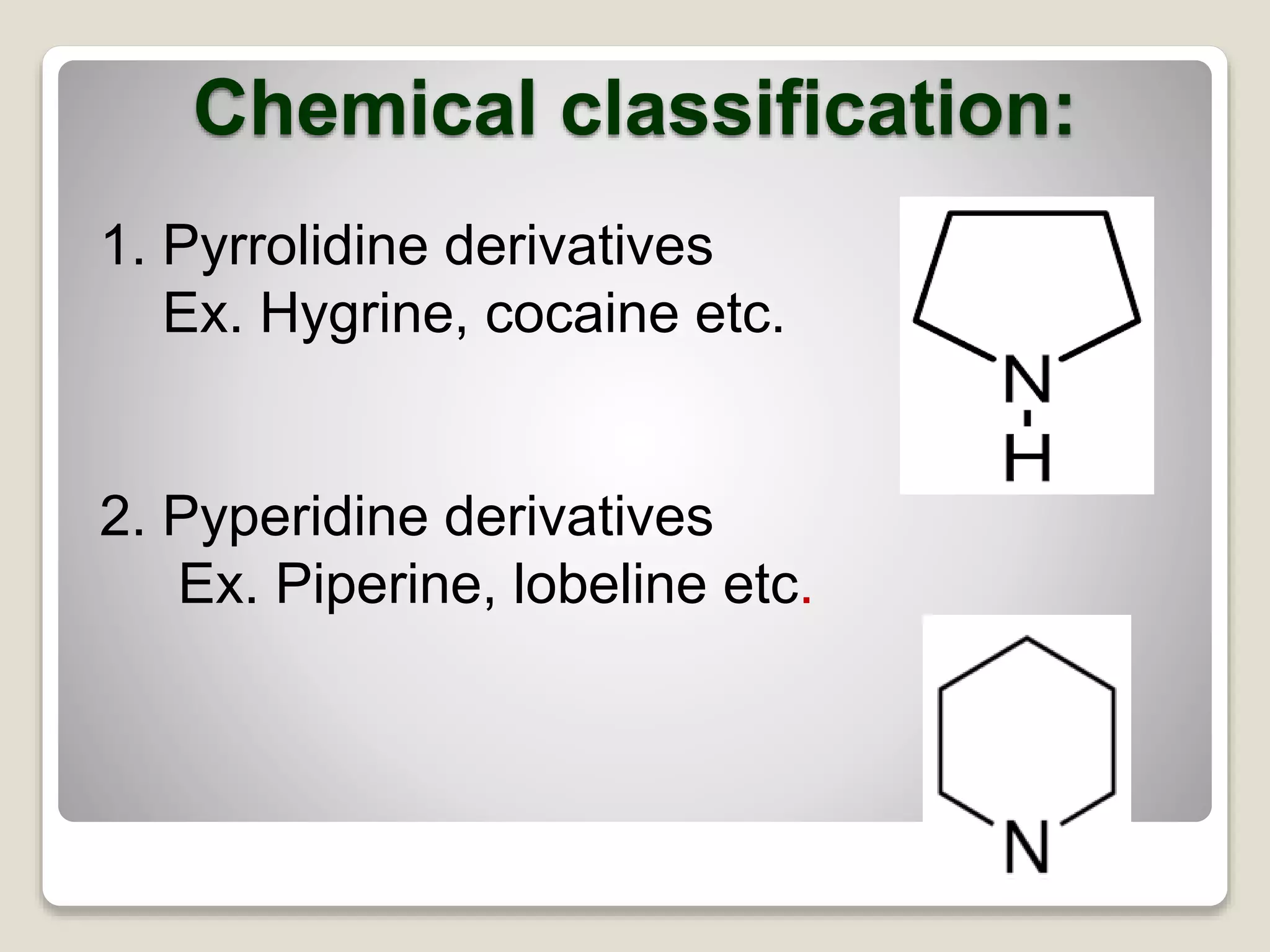
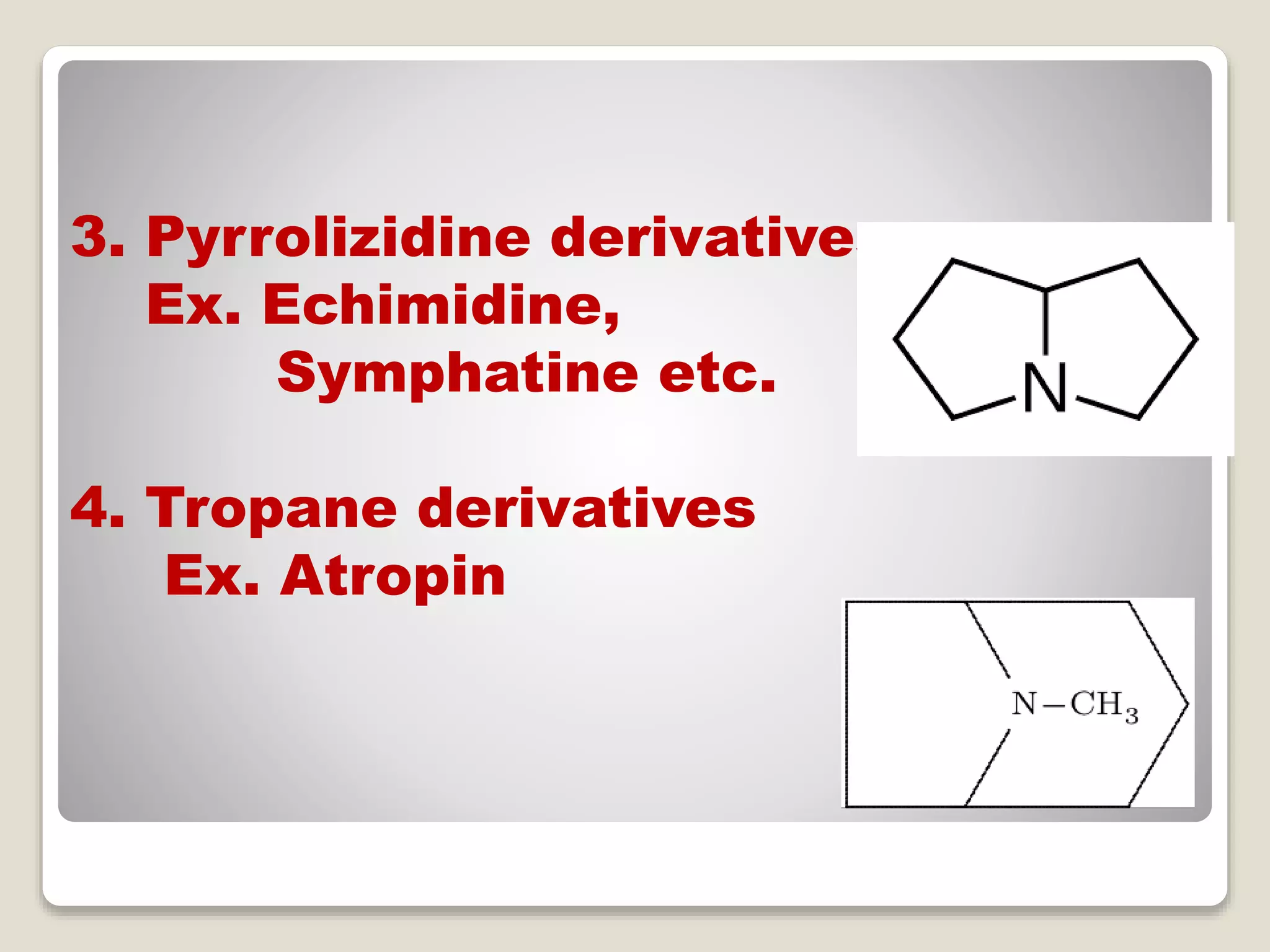
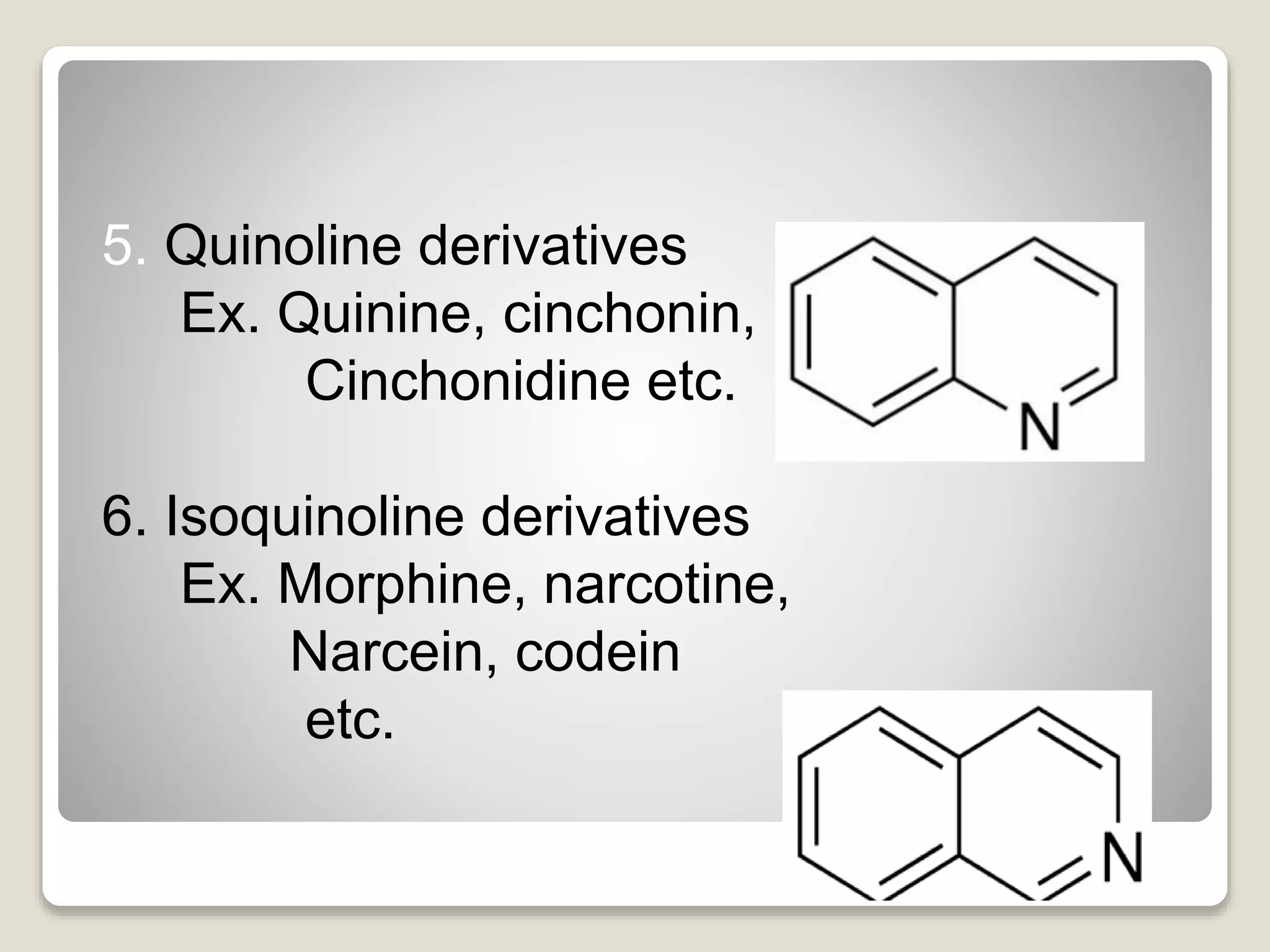
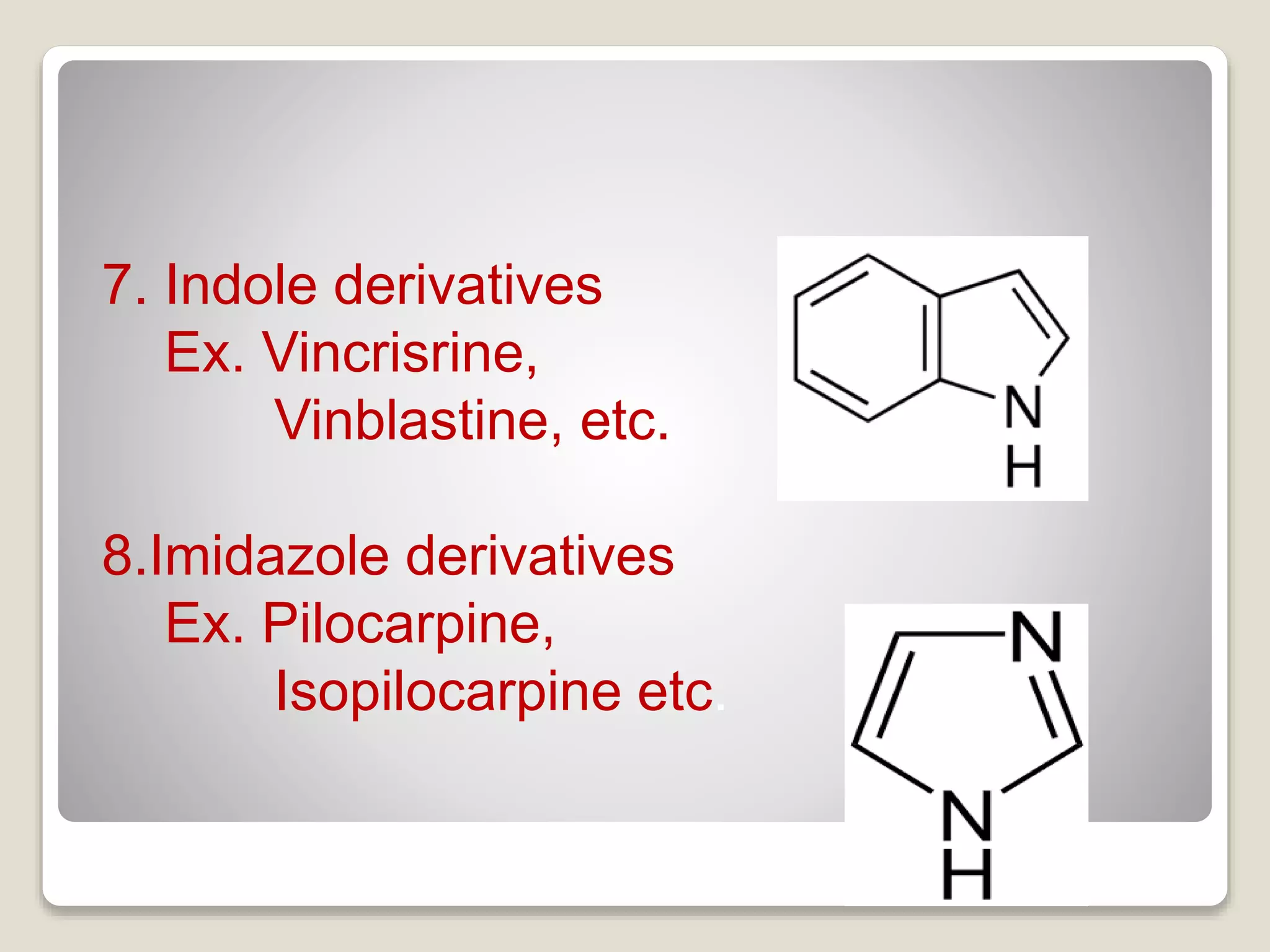
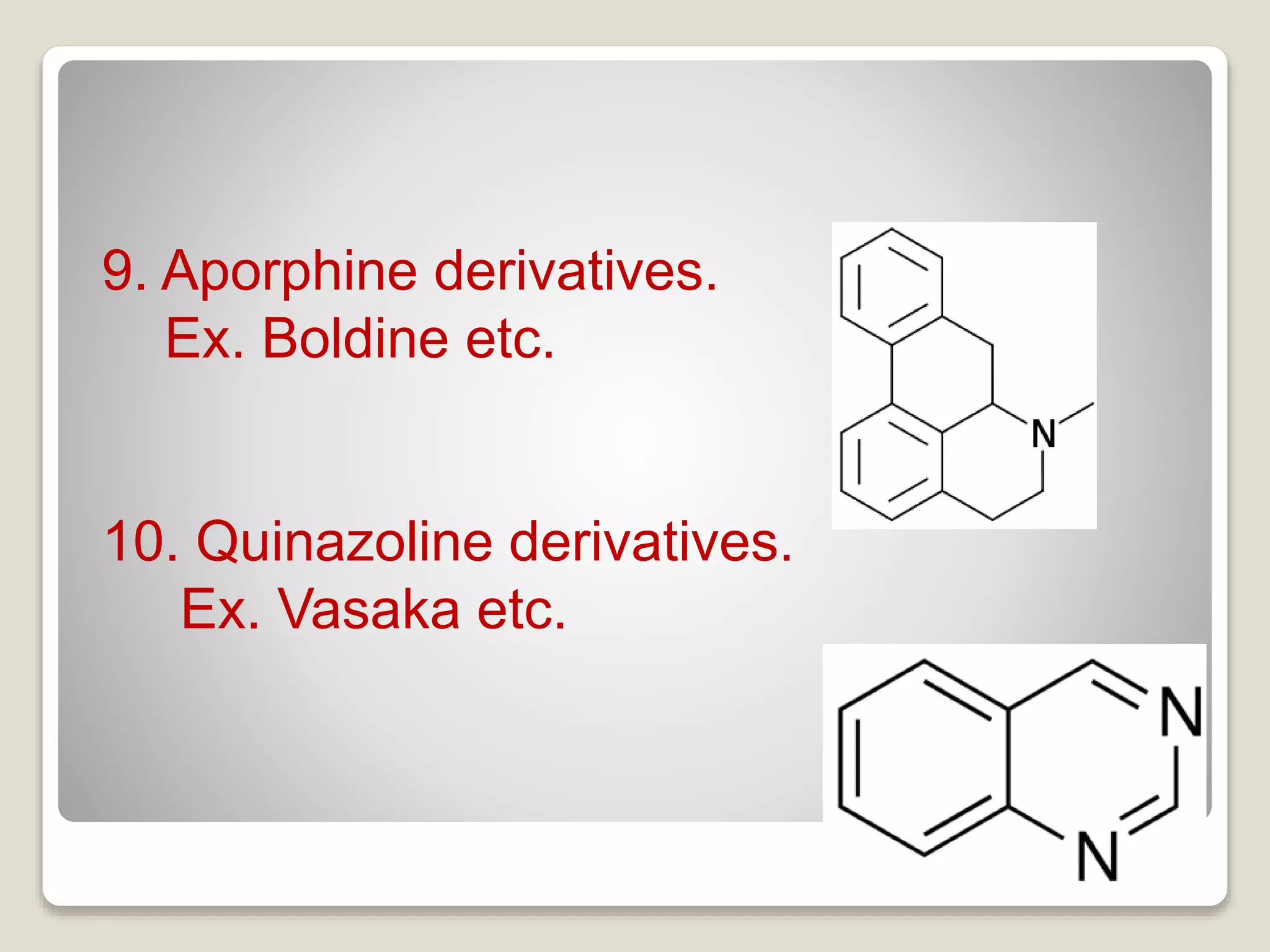
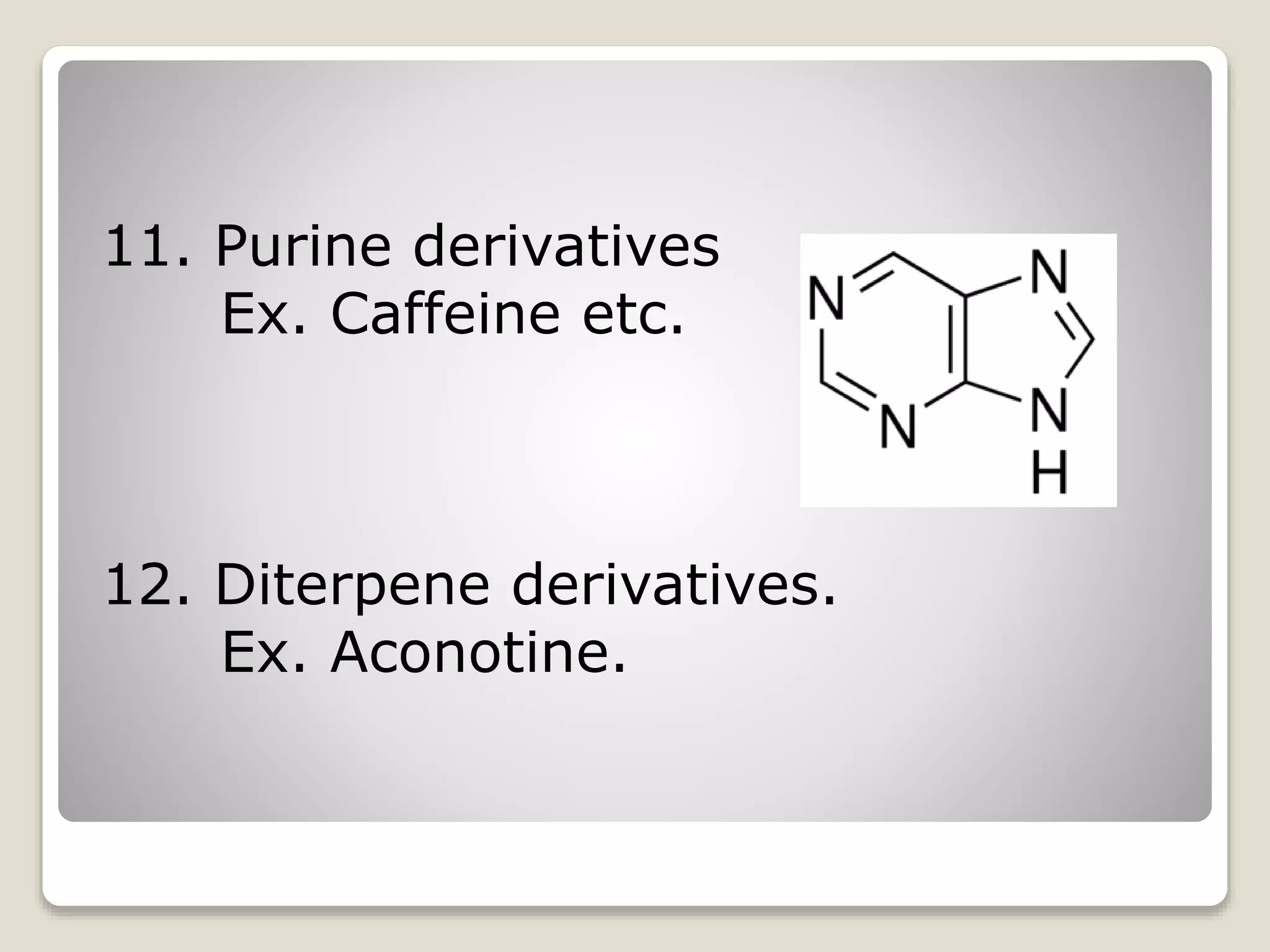
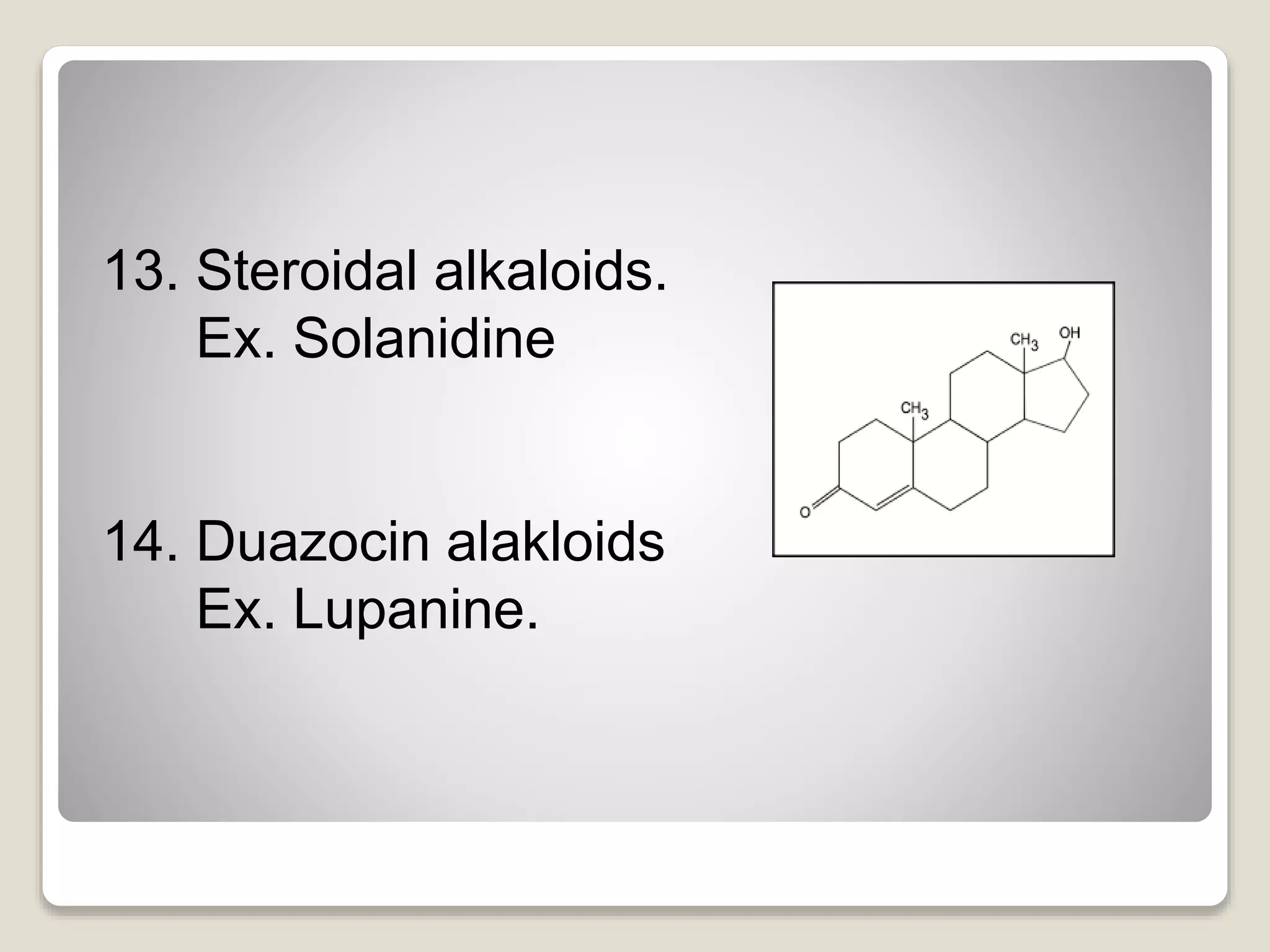
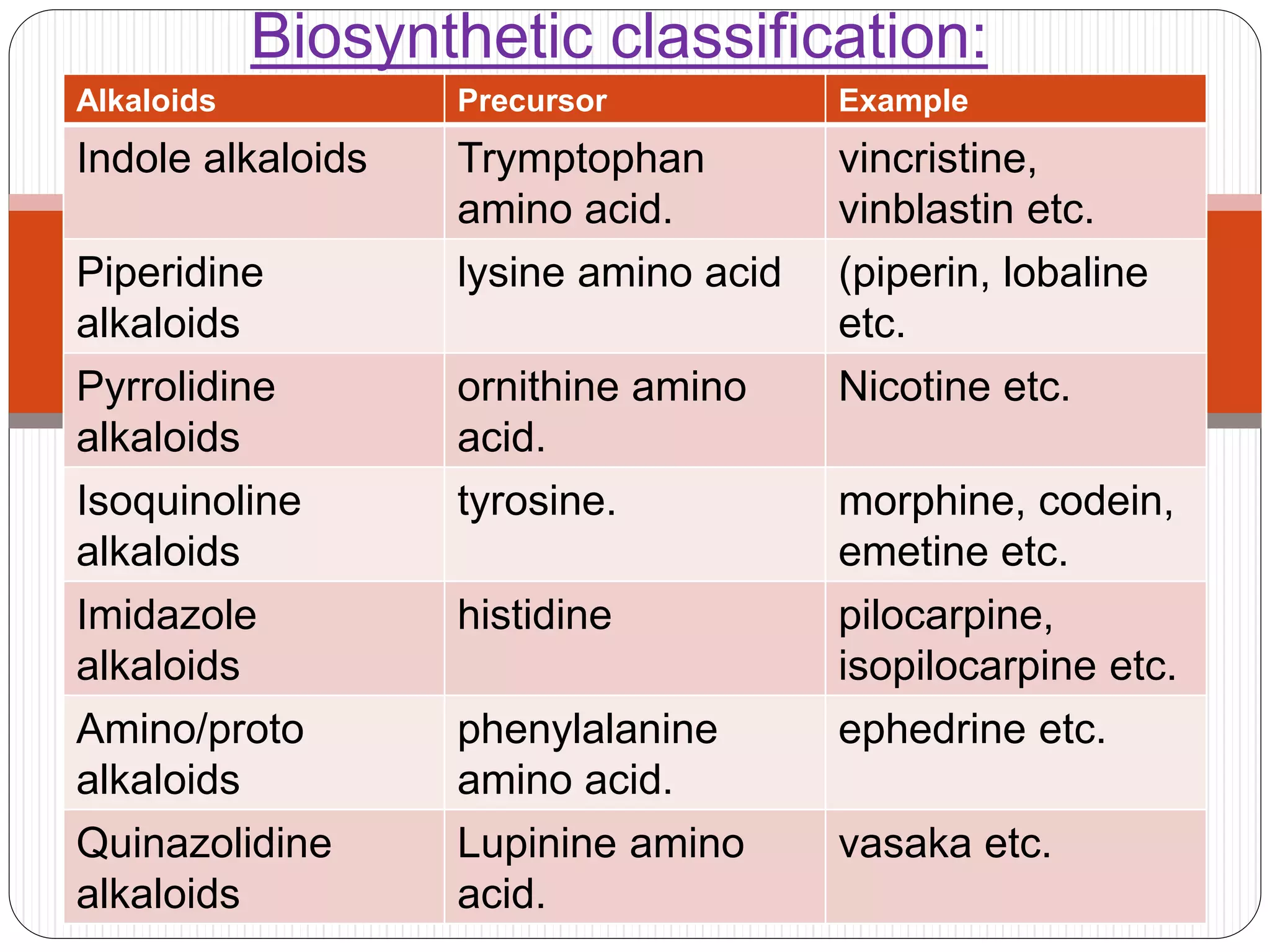
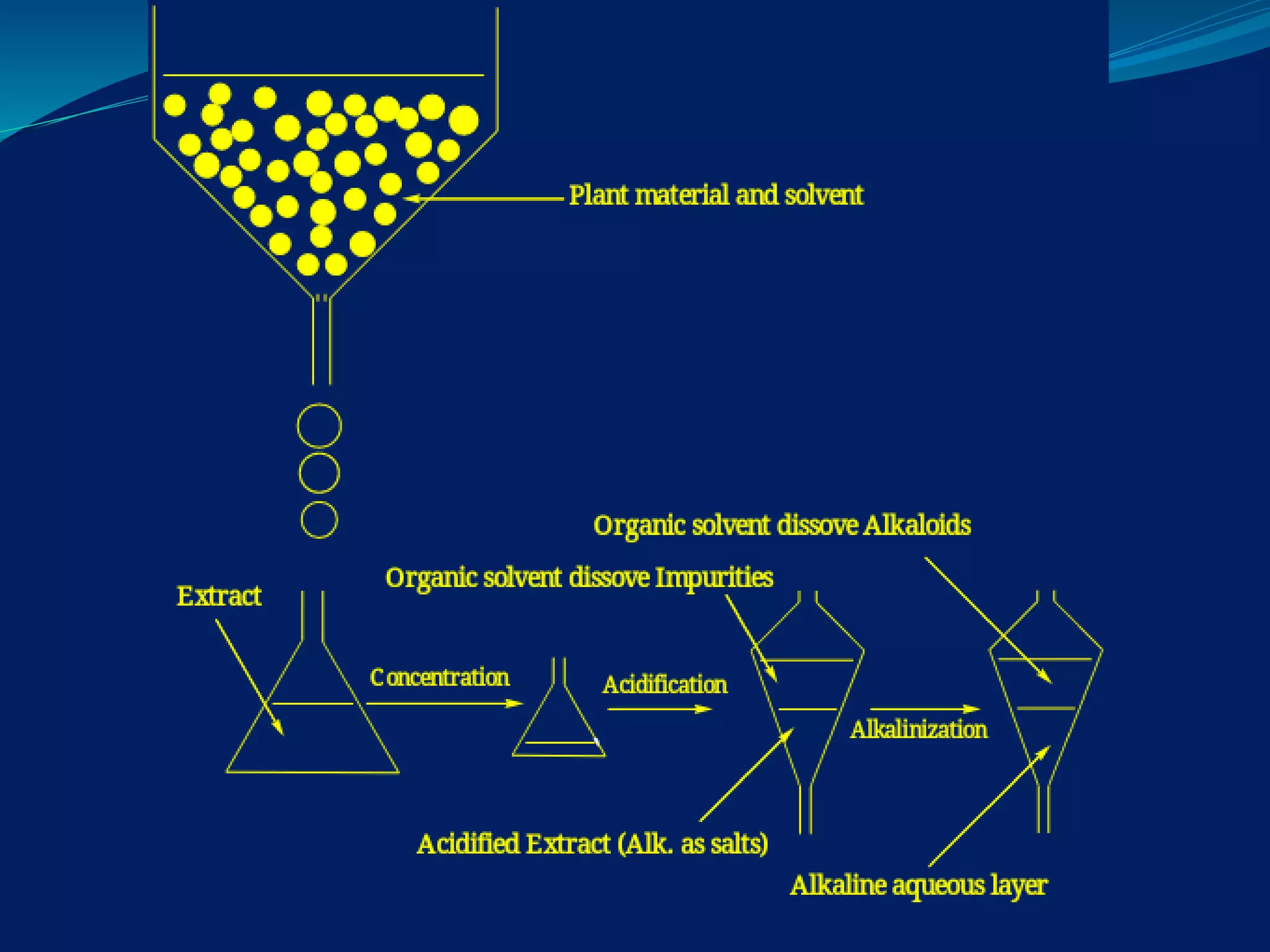
The document provides a comprehensive overview of alkaloids, including their definitions, classifications, physical and chemical properties, and methods of extraction and identification. It distinguishes between primary and secondary metabolites and discusses the specific characteristics of alkaloids, such as their nitrogen content and solubility. Additionally, it details various chemical tests used to detect alkaloids and their biosynthetic classifications based on precursor amino acids.