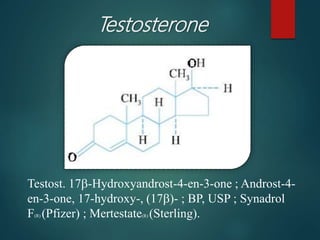
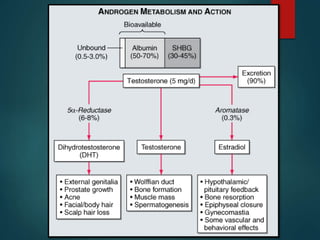
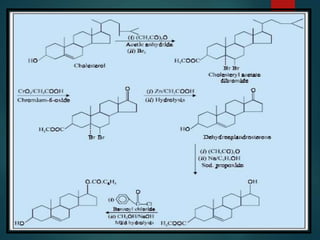
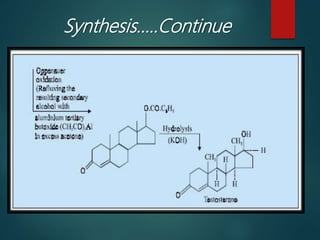
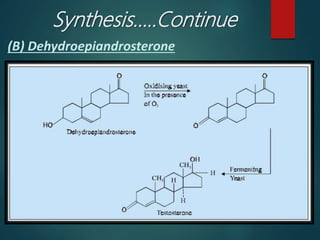
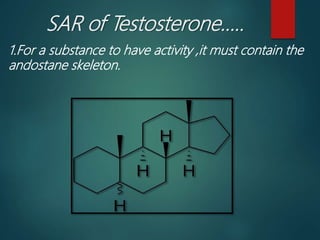
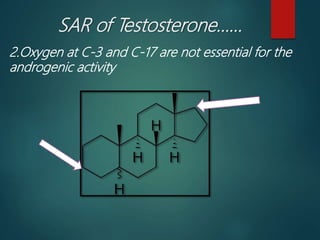
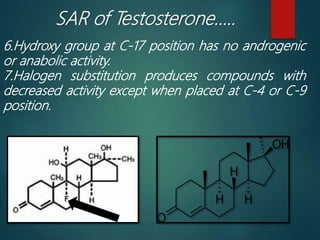
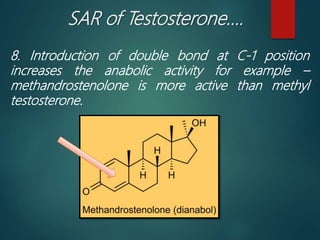
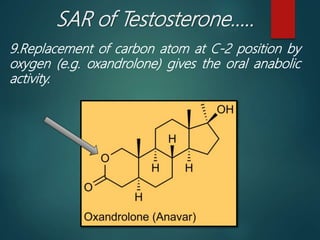
This document discusses testosterone, a male sex hormone. It begins by defining hormones and classifying sex hormones. It then discusses the structure, mechanism of action, synthesis, structure-activity relationships, therapeutic uses, dosing, and adverse effects of testosterone. The synthesis of testosterone is described in multiple steps starting from cholesterol or dehydroepiandrosterone. Testosterone is used to treat hypogonadism and increase muscle mass but can cause masculinization in females and side effects like fluid retention.