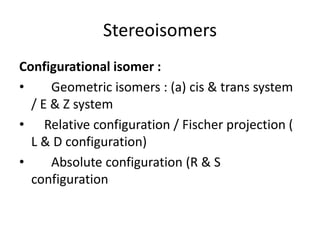
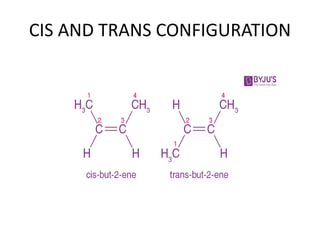
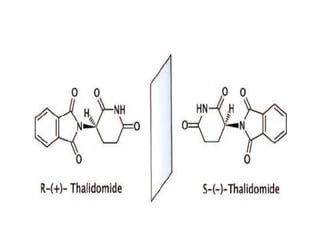
The document discusses stereochemistry and its significance in drug action, focusing on isomerism and chirality in molecules. It explains the concepts of stereoisomers, enantiomers, and diastereomers, highlighting the importance of chiral drugs in pharmacology and their distinct physical and chemical properties. The text also cites examples of chiral drugs and their effects, along with the implications of stereoselectivity on drug metabolism and pharmacokinetics.