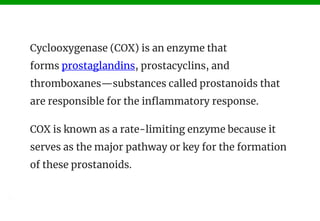
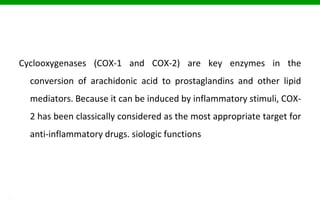
The document discusses COX-1 and COX-2 inhibitors. It notes that there are two forms of the cyclooxygenase enzyme, COX-1 and COX-2, which are involved in inflammation. COX-1 has beneficial effects and is present in most tissues, while COX-2 is primarily found at sites of inflammation. COX-1 inhibitors selectively inhibit COX-1, while COX-2 inhibitors selectively inhibit COX-2. Many classes of non-steroidal anti-inflammatory drugs act as COX inhibitors, including indoles, fenamates, oxicams, pyrazolones, and anilides. Selective COX-2 inhibitors like cele

























![© Ramaiah University of Applied Sciences
28
Faculty of Pharmacy
Anilides [p-aminophenol Derivatives]
• Introduced as analgesics after the discovery of the powerful
antipyretic activities of aniline
• The anilides are simple acetamides of aniline (Aminobenzene),
which may or may not contain a 4-hydroxy or 4-alkoxy group.
• Anilides do not possess the carboxylic acid functionality and
therefore they are classified as neutral drugs and possess little
inhibitory activity against cyclooxygenase.](https://image.slidesharecdn.com/cox-231007182846-6874d6a3/85/COX-pptx-28-320.jpg)
![© Ramaiah University of Applied Sciences
29
Faculty of Pharmacy
Anilides [p-aminophenol Derivatives]
• They are believed to act as scavengers of hydroperoxide
radicals.
• Acetanilide was first introduced and was found to be too toxic
causing jaundice.
• Phenacetin was withdrawn recently due to Nephrotoxicity.
• Paracetamol remains the only useful member of this group
(introduced in1891)](https://image.slidesharecdn.com/cox-231007182846-6874d6a3/85/COX-pptx-29-320.jpg)








