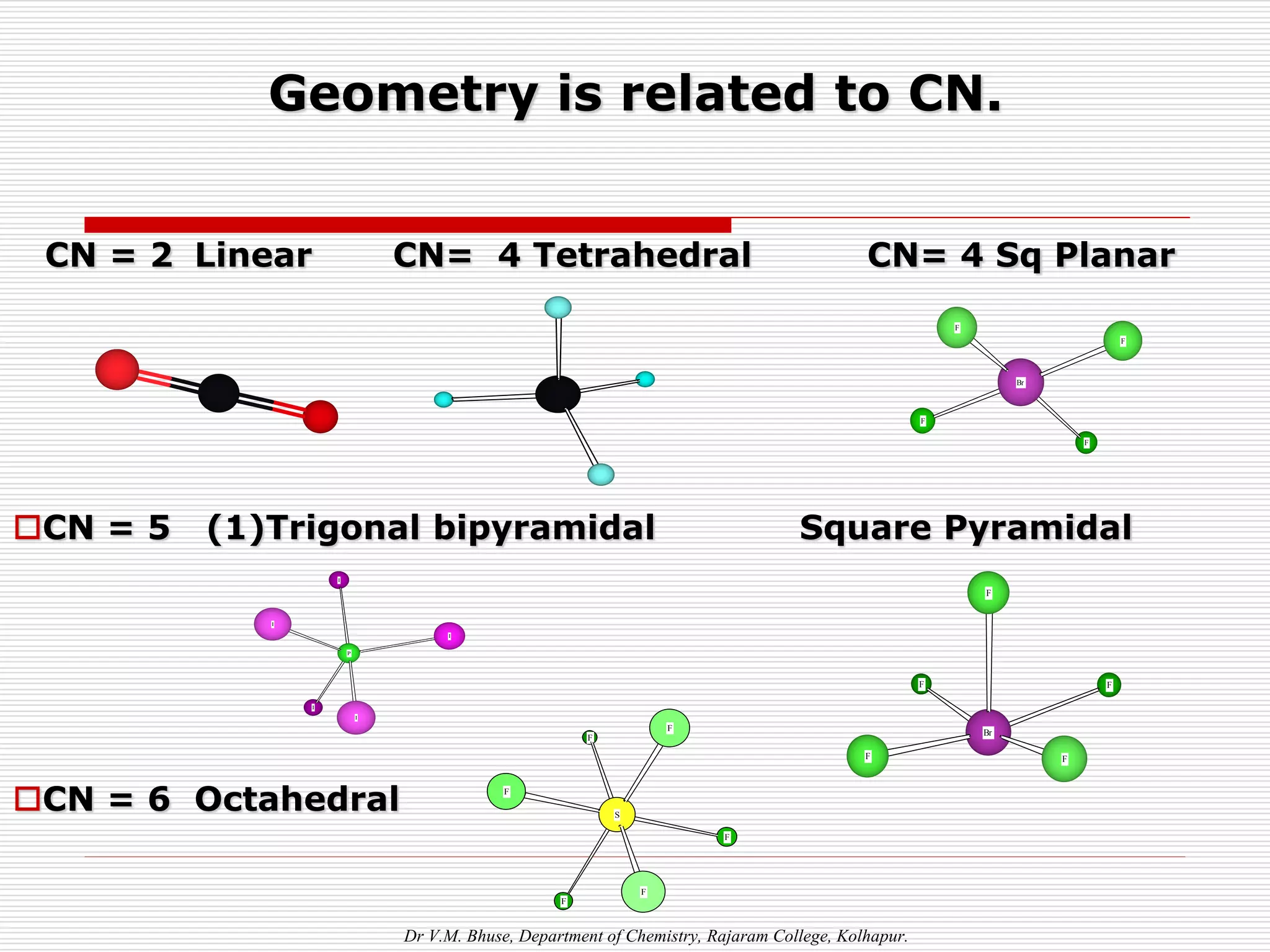
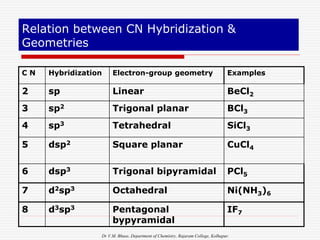
1. The document discusses the relationship between coordination number (CN) and molecular geometry. CN=2 results in linear geometry, CN=3 trigonal planar, CN=4 square planar or tetrahedral, etc.
2. It provides examples of isomers, such as ethanol and acetone which have the same molecular formula but different bond connections.
3. Stereoisomers are isomers that have the same bonding connections but different spatial arrangements, such as cis-trans isomers.

![Dr V.M. Bhuse, Department of Chemistry, Rajaram College, Kolhapur.
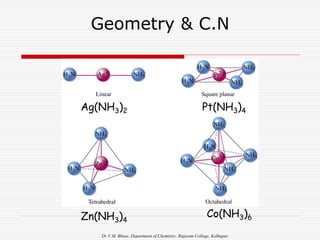
GEOMETRY & CN More examples
CN Shape Example
2 Linear [CuCl2]-, [Ag(NH3)2]+, [AuCl2]-
4 Square Planar [Ni(CN)4] 2-, [PdCl4]2-
[Pt(NH3)4] 2+, [Cu(NH3)4] 2+
4 Tetrahedral [Cu(CN)4] 3-, [Zn(NH3)4]2+
[CdCl4] 2-, [MnCl4] 2-
6 Octahedral [Cu(H2O)6] 3+, [V(CN)6] 4-,
[Cu(NH3)4Cl2] +, [Co(en)3] 3+
F
F
Br
F
F
F
F
F
S
F
F
F](https://image.slidesharecdn.com/coordnisomersb-230905133035-25c53116/85/COORDN-ISOMERS-B-Sc-II-ppt-5-320.jpg)






![Chapter Ten
Prentice Hall © 2005 General Chemistry 4th edition, Hill, Petrucci, McCreary, Perry
Geometrical isomers in Square Planar: (CN = 4)
Examples:
1. Ma2bc
or Mab2c
or Mabc2 [where, M=Pt, a= NH3, b= Py, c=H2O
]
L3 within 90:CIS L3 within 180:TRANS](https://image.slidesharecdn.com/coordnisomersb-230905133035-25c53116/85/COORDN-ISOMERS-B-Sc-II-ppt-13-320.jpg)
![Chapter Ten
Prentice Hall © 2005 General Chemistry 4th edition, Hill, Petrucci, McCreary, Perry
Geometrical isomers in Square Planar: (CN = 4)
Examples
2. Ma2b2
a a
M
b b
a b
M
b a
Trans:Ma2b2
Cis: Ma2b2
[e.g.M= Pt, Pd,A=Cl,Br,I , B=NH3, Py]](https://image.slidesharecdn.com/coordnisomersb-230905133035-25c53116/85/COORDN-ISOMERS-B-Sc-II-ppt-14-320.jpg)

![Chapter Ten
Prentice Hall © 2005 General Chemistry 4th edition, Hill, Petrucci, McCreary, Perry
Geometrical isomers in Square Planar: (CN = 4)
Examples
3.Mabcd
a c
M
d b
Cis: Trans:
a b
M
d c
[e.g. M =Pt,a= Py, b= NH3,c=Cl, d=Br ]
In such example, we have to consider any 2 ligand
same (e.g. a & c same)](https://image.slidesharecdn.com/coordnisomersb-230905133035-25c53116/85/COORDN-ISOMERS-B-Sc-II-ppt-16-320.jpg)




















