1) Mississippi State University is researching pyrolysis of giant miscanthus to produce biofuels such as bio-oil, hydrocarbons, and ethanol.
2) Their auger reactor design can produce bio-oil at 67% yield from giant miscanthus and the design is being licensed to an industrial partner for commercialization.
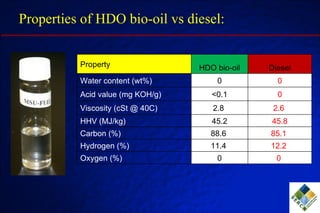
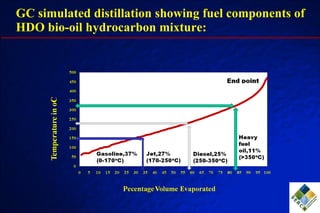
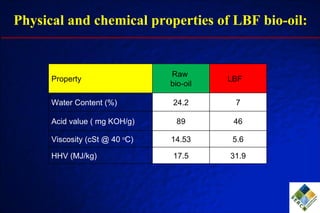
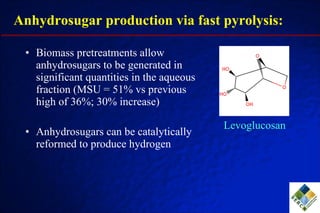
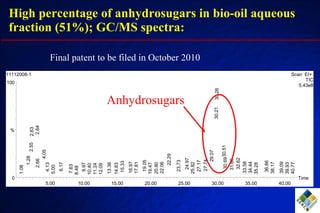
3) Through hydrodeoxygenation and esterification, they can upgrade bio-oil into hydrocarbon mixtures and boiler fuel with properties similar to diesel, as well as produce anhydrosugars from the aqueous fraction that can be converted to ethanol.