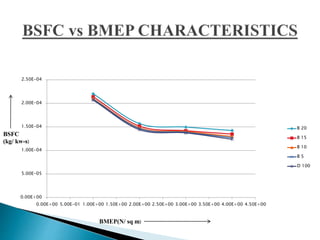
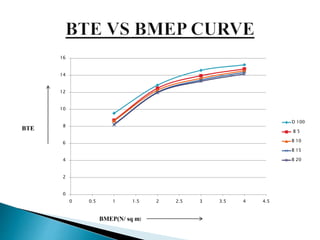
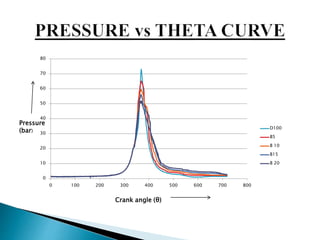
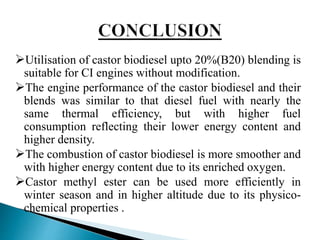
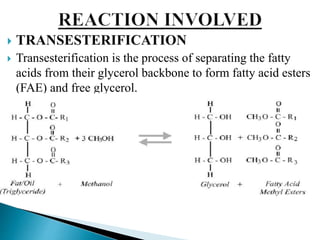
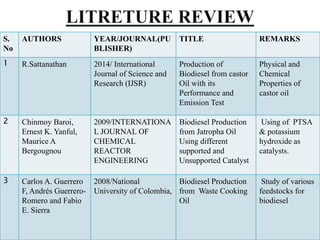
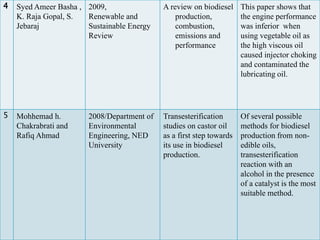
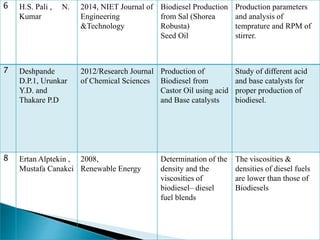
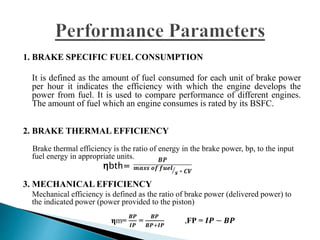
The document presents a project on producing biodiesel from castor oil and testing its performance in a single cylinder diesel engine. It discusses castor oil properties, biodiesel production process including transesterification reaction, literature review on biodiesel research, objectives to produce biodiesel from castor oil and measure engine performance, and conclusions from test results showing biodiesel blends up to 20% can be used without modifications.










![4 . INDICATED THERMAL EFFICIENCY
Indicated thermal efficiency is the ratio of energy in the indicated
power, IP, to the input fuel energy in appropriate units.
ηth =
𝒊𝒏𝒅𝒊𝒄𝒂𝒕𝒆𝒅 𝒑𝒐𝒘𝒆𝒓 [
𝒌𝒋
𝒔]
𝒆𝒏𝒆𝒓𝒈𝒚 𝒊𝒏 𝒇𝒖𝒍 𝒑𝒆𝒓 𝒔𝒆𝒄𝒐𝒏𝒅 [
𝑲𝒋
𝒔]
5. MEAN EFFECTIVE PRESSURE
Mean effective pressure is the average pressure inside the cylinder of an
internal combustion engine based on the calculated or measured power output. It
increases as manifold pressure increases. For any particular engine, operating at a
given speed and power output, there will be a specific indicated mean effective
pressure, imep, and a corresponding brake mean effective pressure.](https://image.slidesharecdn.com/castorbiodieselnwppt-150617061549-lva1-app6892/85/Castor-biodiesel-24-320.jpg)