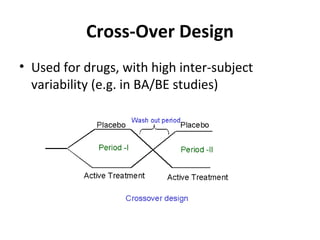
The document discusses key concepts in clinical trial designs, including types of trials, phases of trials, and protocol requirements according to ICH-GCP guidelines. It describes the various types of clinical trial designs such as treatment, prevention, diagnostic, and screening trials. It also outlines the different phases of clinical trials from phase I to phase IV and summarizes the key elements that must be included in a clinical trial protocol according to ICH-GCP, such as the trial design, randomization, blinding, treatment regimens and stopping rules.