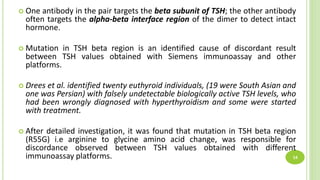
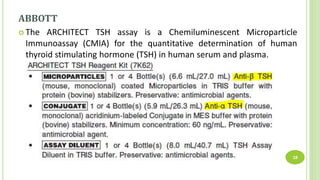
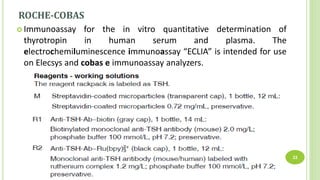
This document reports a case of a young Nepalese male who was initially misdiagnosed with subclinical hyperthyroidism due to an undetectable TSH level, but was later found to likely have a TSH variant. The patient's TSH was undetectable on the Siemens Advia Centaur XP immunoassay system but showed a normal level on other platforms. The authors discuss that mutations in the TSH beta region can cause discordant results between different immunoassay systems. Testing the patient's serum on multiple platforms found the TSH level was normal on Roche and Abbott systems, suggesting a TSH variant as the cause rather than hyperthyroidism. Physicians should be aware of potential immunoassay