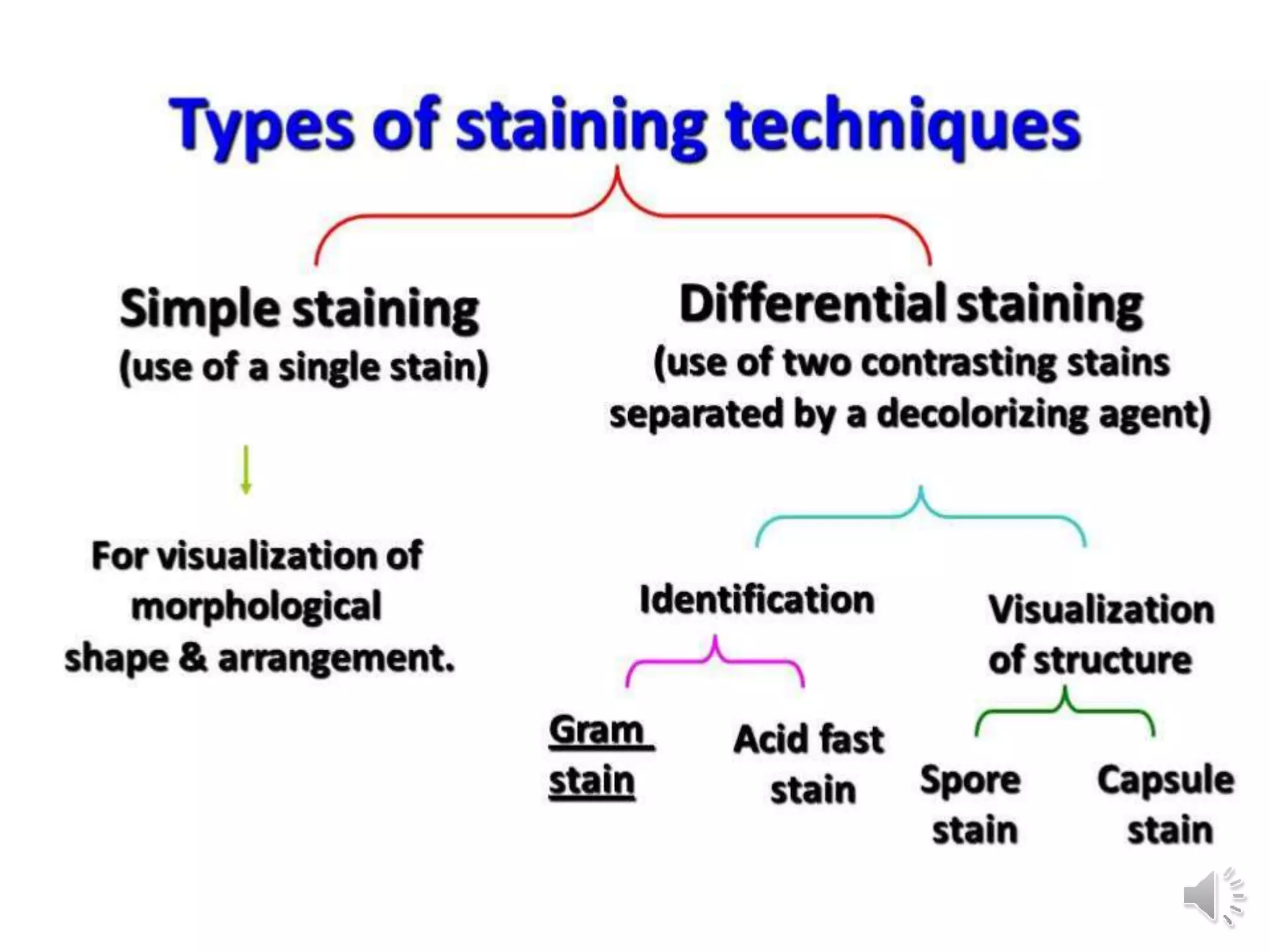
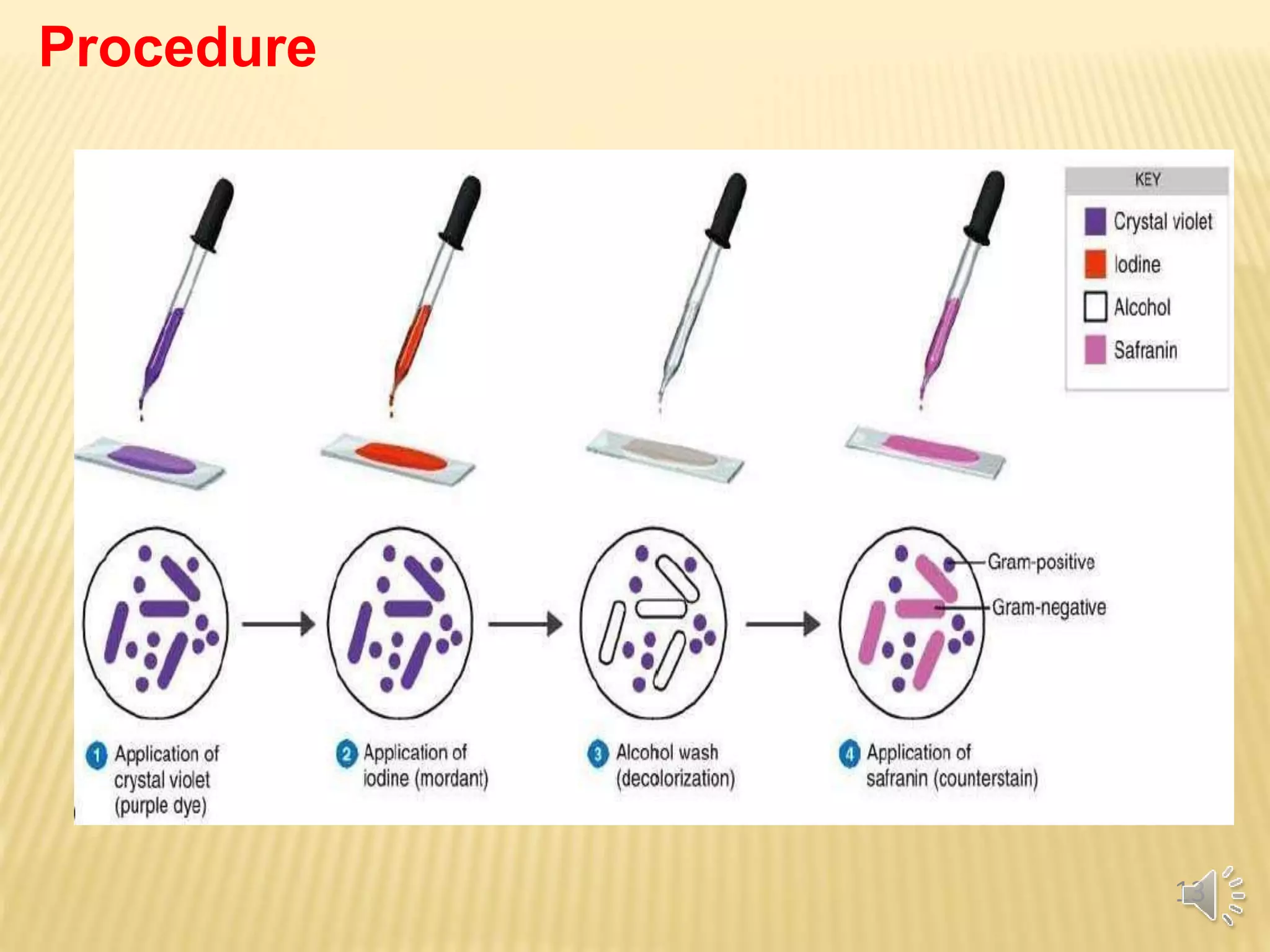
The document discusses various staining techniques used to visualize bacteria for study purposes, detailing positive and negative staining methods. It covers specific methods such as Gram staining, acid-fast staining, and structural stains for endospores, capsules, and flagella, providing principles and procedures for each. Additionally, it highlights the importance of chemical properties in selecting appropriate stains and techniques for accurately observing bacterial characteristics.