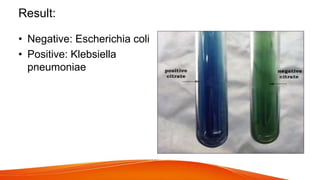
The citrate utilization test is used to differentiate between members of the Enterobacteriaceae family based on their ability to use citrate as a sole carbon source. Some bacteria like Enterobacter and Klebsiella are able to convert citrate to oxaloacetate and acetate through metabolic pathways that produce carbon dioxide. The test uses Simmon's citrate agar with citrate as the sole carbon source and a pH indicator. A color change in the medium from orange to blue indicates positive test results and the ability to use citrate. E. coli tested negative while Klebsiella pneumoniae tested positive on the citrate utilization test.