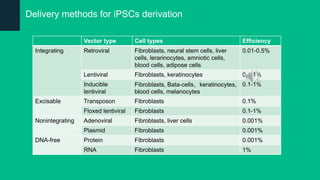
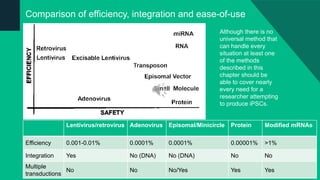
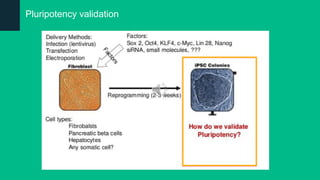
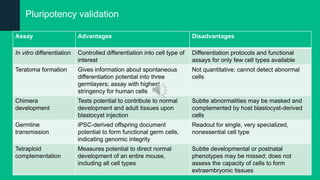
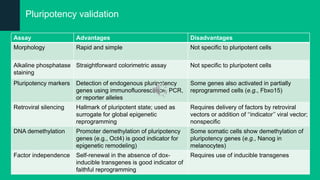
The document discusses induced pluripotent stem cells (iPSCs), emphasizing their advantages over embryonic stem cells (ESCs) such as avoiding ethical concerns and facilitating personalized medicine. It details methods for accessing iPSCs, including various reprogramming factors and delivery methods, along with their efficiencies and challenges. Additionally, it examines methods for validating pluripotency and the potential and limitations of iPSCs in biomedical research and disease modeling.