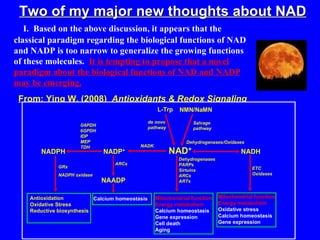
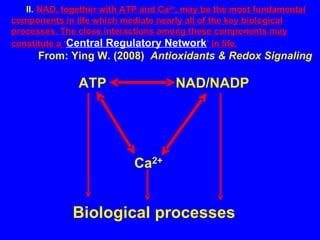
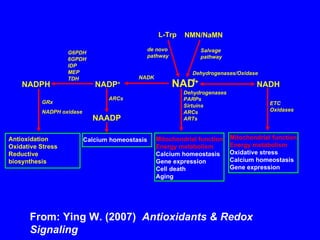
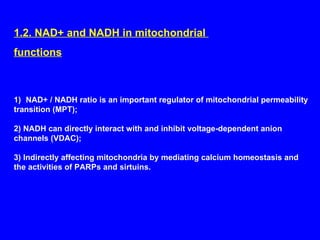
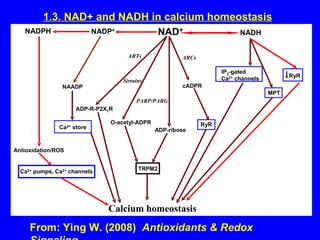
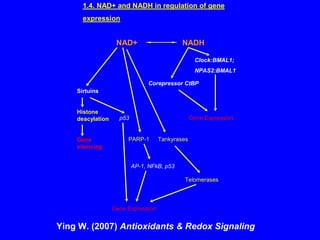
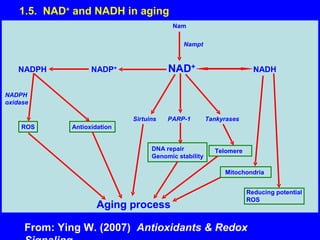
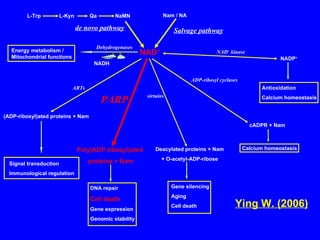
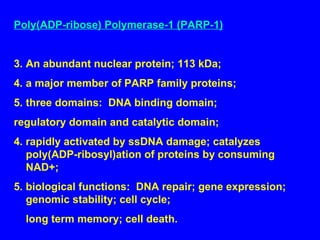
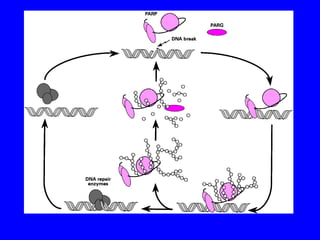
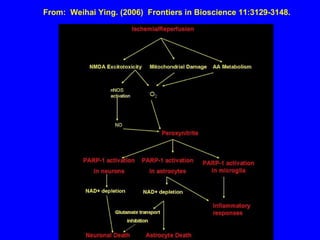
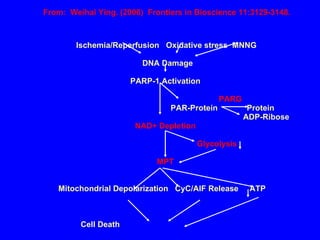
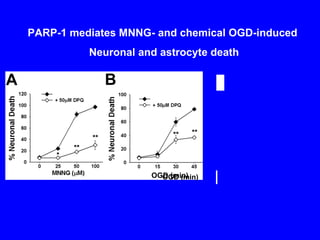
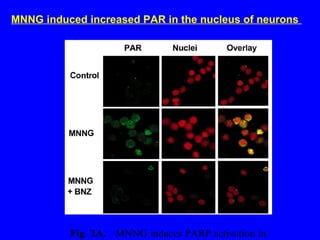
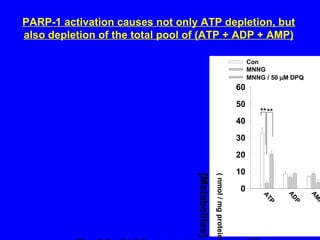
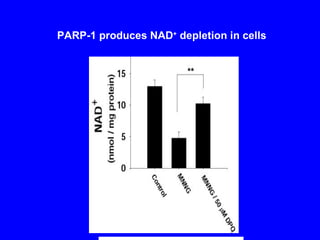
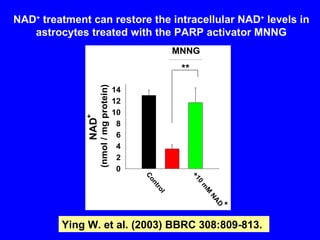
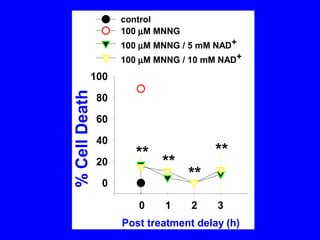
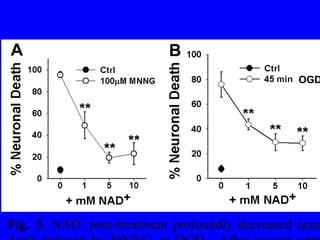
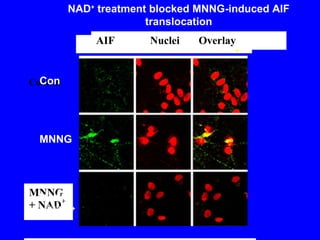
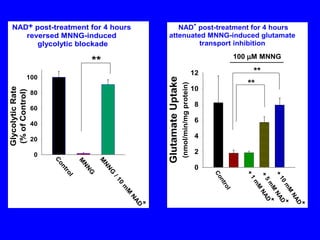
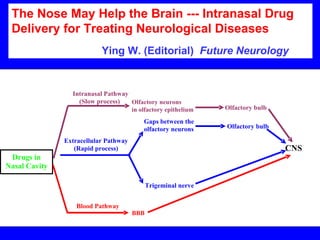
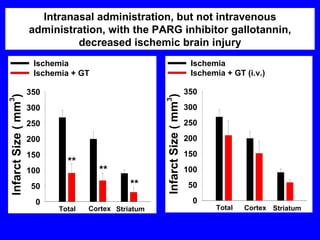
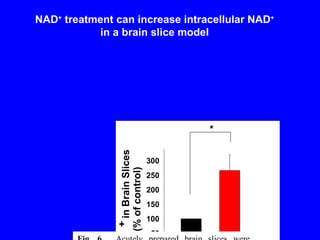
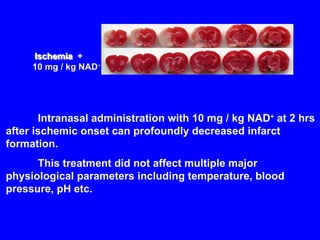
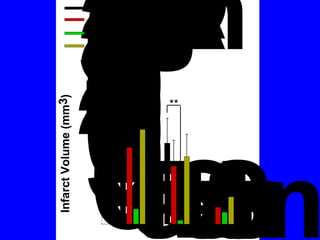
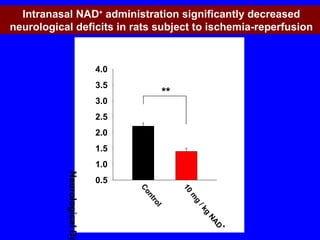
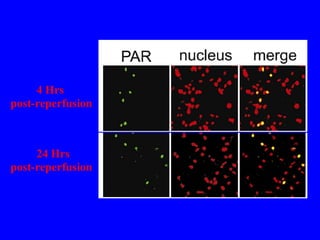
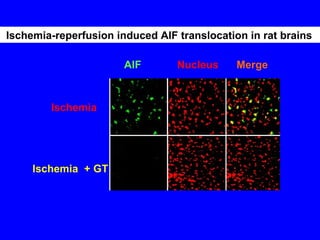
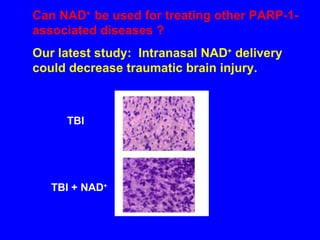
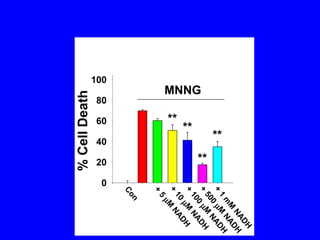
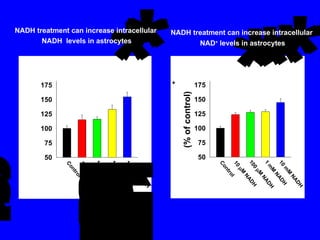
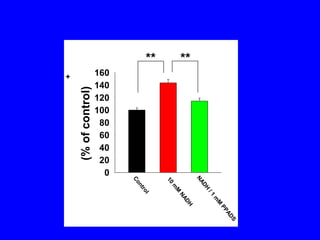
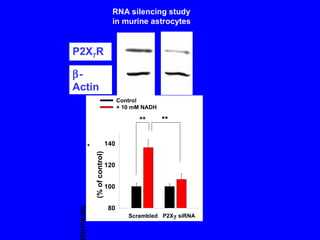
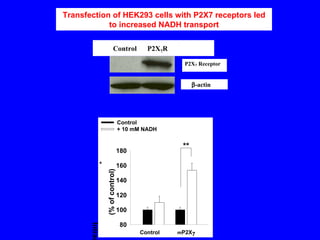
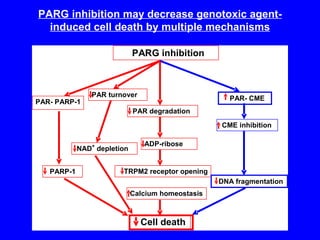
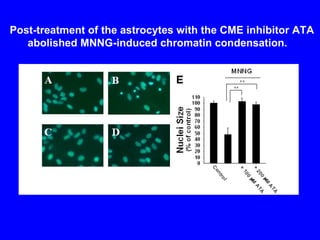
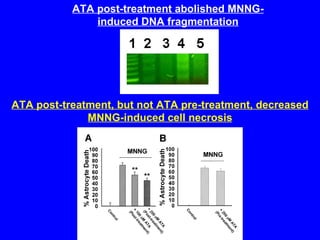
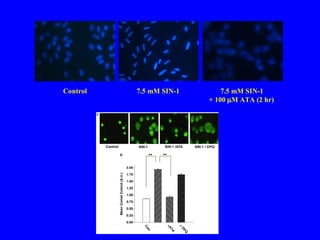
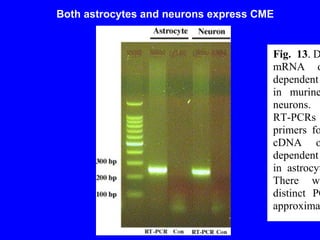
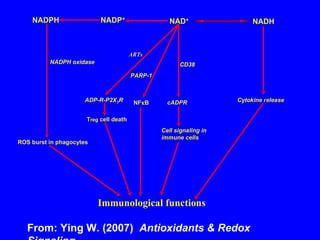
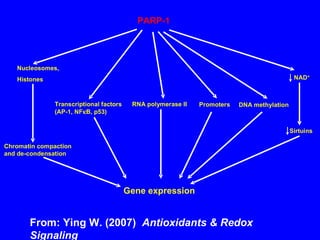
NAD+ and NADH play roles in many important biological processes such as energy metabolism, mitochondrial function, calcium homeostasis, and gene expression. They have emerged as one of the most influential couples in life. Poly (ADP-ribose) polymerase 1 (PARP1) activation leads to NAD+ depletion, which mediates cell death. Intranasal administration of NAD+ can decrease ischemic brain injury by reducing PARP1-induced cell death and is a potential treatment for diseases involving PARP1.